Authorship
Shaw, Nancy; Gucker, Corey
Publication Date
February 2020
Nomenclature
Rocky Mountain beeplant (Peritoma serrulata [Pursh] de Candolle) is a member of the Cleomaceae or spiderflower family (Vanderpool and Iltis 2010) but was formerly placed in family Capparaceae. The earliest specimen was collected in 1804 by Meriwether Lewis along the Missouri River near Vermillion in Clay County, South Dakota (Reveal et al. 1999). Recent molecular work leaves the taxonomic placement of the family, genus, and species in question (see Hall 2008; Iltis et al. 2011; Roalson et al. 2015).
Family
Cleomaceae – Spiderflower family
Genus
Peritoma [Cleome]
Species
serrulata
NRCS Plant Code
PESE7, CLSE (USDA NRCS 2020).
Subtaxa
No subspecies or varieties are recognized by the Flora of North America (Vanderpool and Iltis 2010). Welsh et al. (2015), using the synonym Cleome serrulata, recognized two intergrading phases in Utah: C. s. (Pursh) var. serrulata, which is widespread and C. s. var. angusta (M. E. Jones) Tidestrom, which occurs only in Utah’s southern counties.
Synonyms
Cleome serrulata Pursh, C. serrulata subsp. angusta (M. E. Jones), Peritoma inornata (Greene) Greene, P. serrulata var. albiflora Cockerell, P. serrulata var. clavata Lunell (Vanderpool and Iltis 2010).
Common Names
Rocky Mountain beeplant, a’ pilalu (Zuni name), bee spiderflower, guaco, Navajo spinach, pink cleome, pink bee plant, skunk weed, stinkweed, stinking clover, toothed spider-flower, ‘wahpe’ – h’eh’e (Lakota name) (Stevenson 1915; Craighead et al. 1963; Harrington 1967; Rogers 1980; Currah 1983; Kindscher 1987; Blackwell 2006; Vanderpool and Iltis 2010; Winslow 2014; PFAF 2019). The common names derive from the many pollinators, primarily bees, attracted to the plant; its profuse nectar production; the resemblance of the long, dangling pods to the legs of a spider; the plant’s slightly unpleasant odor; and its widespread use by Native Americans.
Chromosome Number
2n = 16, 17, 32, 34, 60 (Holmgren and Cronquist 2005; Vanderpool and Iltis 2010; Rice et al. 2015; Welsh et al. 2015).
Hybridization
Holmgren and Cronquist (2005) found no evidence of hybridization. Welsh et al. (2015), however, report occasional hybrids between C. s. var. angusta and C. lutea.
Distribution
Rocky Mountain beeplant is widespread in the West. It occurs in the Canadian Provinces of British Columbia, Alberta, Manitoba, Ontario, and Saskatchewan. In the United States it is found primarily east of the Cascade and Sierra Nevada Mountains from Washington to California, Arizona, New Mexico, and the northeastern portion of the Texas Panhandle (Iltis in Corell and Johnston 1979; Vanderpool and Iltis 2010; LBJWC 2019). Rocky Mountain beeplant is found in 43 of the 56 counties in Montana, 21 of Wyoming’s 23 counties, and all Utah counties (Lesica et al. 2012; Winslow 2014; Welsh et al. 2015). It grows on the Colorado Plateau and in Arizona mountains and sky islands (The Xerces Society and USDA NRCS 2012). In New Mexico, it is common in the mountains but absent near the state’s southern and eastern borders (Iltis 1958). Collections from the Midwest and Northeast may have escaped from gardens (Holmgren and Cronquist 2005).
Habitat And Plant Associations
Rocky Mountain beeplant grows in sun or part shade on a variety of soil types in shortgrass and mixed-grass prairies, sagebrush (Artemisia spp.) steppe, dry meadows, desert scrub, pastures, and pinyon pine (Pinus spp.) and juniper (Juniperus spp.) woodlands (Iltis 1958; Blackwell 2006; Vanderpool and Iltis 2010). It is common in low elevation valleys; dry, sandy plains; mountain foothills; and barren rangeland. A pioneer plant, it often occurs on disturbed sites that are sparsely vegetated (e.g., dry washes, ditch banks, banks and terraces of natural water courses, coulee edges, and along roadsides; Fig. 1), but it is not tolerant of water logging (Iltis 1957; Craighead et al. 1963; Weber 1976; Kindsher 1987; Andersen and Holmgren 1996; Spellenberg 2001; Lesica et al. 2012; Ogle et al. 2012; Winslow 2014; Welsh et al. 2015).
In Montana and Wyoming, it often grows in association with western wheatgrass (Pascopyrum smithii), bluebunch wheatgrass (Pseudoroegneria spicata), prairie June grass (Koeleria cristata), Sandberg’s bluegrass (Poa secunda), blanketflower (Gaillardia aristata), upright prairie coneflower (Ratibida columnifera), and big sagebrush (A. tridentata) (Winslow 2014). Beatley (1976) reported it in lowland shadscale (Atriplex confertifolia) vegetation on basin floors in closed drainage basins at elevations of 5,000 to 5,200 ft (1,524–1,585 m) in Nevada.


Figure 1. Rocky Mountain beeplant growing in Utah along a roadside (top) and in the Owyhee mountains of Idaho (bottom). Photos: USDI BLM UT080 SOS (top); C. Shock (bottom).
Elevation
Rocky Mountain beeplant grows at elevations from (330) 980 to 8,200 (9,500) ft ([100] 300–2,500 ft [2,900] m) (Vanderpool and Iltis 2010). It is found at elevations of 2,625 to 9,006 ft (800–2,745 m) in Utah (Welsh et al. 2015), 4,000 to 7,200 ft (1,200–2,200 m) in Wyoming, and 2,500 to 5,200 ft (760–1,585 m) in Montana (Winslow 2014). On the Colorado Plateau, Arizona Mountains and sky Islands, it grows at 4,500 to 7,000 ft (1,400–2,100 m) (The Xerces Society and USDA NRCS 2012).
Soils
Rocky Mountain beeplant is most common on well-drained, moist to dry, medium-textured to sandy soils with pH 6 to 7.6 (Currah et al. 1983; Kindscher 1987; PFAF 2019; USDA NRCS 2020), but it also occurs on clay and gravel soils (Iltis 1958). It grows on sites with at least 70 frost-free days and tolerates drought and moderate amounts of calcium carbonate (Winslow 2014; LBJWC 2019; USDA NRCS 2020). P. s. subsp. angusta commonly occurs on sandy soils and is more drought tolerant than P. s. subsp. serrulata (R. Stevens, Utah Division of Wildlife Resources [retired], personal communication, February 2020).
Description
Rocky Mountain beeplant is a tall, upright, somewhat malodorous, colonizing annual plant growing 1 to 6.6 ft (0.3–2 m) tall with a 12– to 14–in (30- to 35-cm) taproot. It produces dense racemes of purple to sometimes white flowers and elongate, spreading pod-like capsules (Fig. 2) (Currah 1983; Cane 2008; Welsh et al. 2015). Plants are erect with glabrous to glabrate stems that may branch above, creating an open, rangy structure. Leaves are alternate, glabrous or with marginal hairs when young, and palmate with three entire to slightly sinuate or serrulate leaflets 0.8 to 2.4 in (2–6 cm) long and 0.2 to 0.6 in (0.6–1.5 cm) wide. Leaflets range from lanceolate to elliptic or oblanceolate with mucronate to long acuminate tips (Holmgren and Cronquist 2005; Vanderpool and Iltis 2010; Welsh et al. 2015). Petioles have bristle-like stipules (Vanderpool and Iltis 2010).

Figure 2. Rocky Mountain beeplant exhibits a rangy growth structure with branches developing from the upper part of the stem, dense inflorescences, and spreading capsules. Photo: Matt Lavin, Montana State University.
Flowers develop in terminal, densely crowded, bracteate racemes (Fig. 3) that are 0.4 to 1.6 in (1–4 cm) long, elongating to 1.6 to 11.9 in (4–30 cm) in fruit. Bracts are simple and much smaller than the leaves (Welsh et al. 2015). Flowers are regular to irregular with green to purple pedicels. Sepals are four, broad, persistent, dentate, and connate to 1/2 to 2/3 of their length (Welsh et al. 2015). The four narrow petals are commonly purple, but range to pink or white. They are oblong to ovate and 2.8 to 4.7 in (7–12 cm) long and 0.1 to 0.2 in (3–6 mm) wide, narrowing abruptly near the base. Buds of Rocky Mountain beeflower are a deeper color than the open flower. The lighter color of the mature petals may be age related and does not seem to be a pollinator cue (Nozzolillo et al. 2010).
Rocky Mountain beeplant produces perfect and staminate flowers. Perfect flowers have both stamens and a pistil. Staminate flowers begin as perfect buds, but pistil formation is incomplete, thus flowering is not andromonoecious (with staminate and perfect flowers on the same plant) because truly staminate flowers are not produced (Cane 2008). Flowers produce six long, equal, threadlike stamens with purple filaments and green anthers that are exserted as the flower opens (Fig. 4). The pistil of perfect flowers has a basal gland and unilocular ovary with two parietal placentae. The stamens and pistil arise from an androgynophore (stipe) (Blackwell 2006; Vanderpool and Iltis 2010) that is 0.04 to 0.6 in (1–15 mm) long in fruit (Munz and Keck 1973; Holmgren and Cronquist 2005; Vanderpool and Iltis 2010; Welsh et al. 2015).

Figure 3. Densely flowered, indeterminate Rocky Mountain beeplant inflorescence with darker colored buds at the apex and flowers and capsules further down the raceme. Note the exerted stamens with green anthers and pink filaments. Photo: Matt Lavin, Montana State University.
The fruits are dehiscent, striated, glabrous, pod–like capsules 1 to 3 in (2.5– 7.6 cm) long and 0.1 to 0.2 in (3–6 mm) wide, terete in cross section, and pointed at both ends (Fig. 5). The capsules become more separated as the inflorescence elongates (Craighead et al. 1963; Currah et al. 1983; Kindscher 1987; Vanderpool and Iltis 2010). The pedicels are ascending and 0.4 to 0.6 in (10–15 mm) long; the fruiting stipes are more spreading and 0.4 to 0.9 in (11–23 mm) long with the mature fruits pendulous. Mature viable seeds are 2.8–4 mm x 2.5 to 3 mm, dark brown to black, and spherical to ovoid or horseshoe-shaped with rough surfaces (Fig. 5) (Currah et al. 1983; Holmgren and Cronquist 2005; Vanderpool and Iltis 2010; Winslow 2014). Fruit size variation has been attributed to environmental rather than genetic causes (Iltis 1952 in Vanderpool and Iltis 2010). Seeds reportedly lack or contain only small amounts of endosperm (Iltis 1958; Sanchez-Acebo 2005). The embryo is curved and folded (Iltis 1958). Flowering dates depend on the location, but across the species’ range, flowering may occur from May to October (Iltis 1958; Munz and Keck 1973; Holmgren and Cronquist 2005).

Figure 4. Perfect Rocky Mountain beeplant flower with the capsule beginning to develop. Photo: Matt Lavin, Montana State University.


Figure 5. Fruits (capsules) of Rocky Mountain beeplant (top) and horseshoe–shaped seeds with rough surfaces (bottom). Photos: Matt Lavin, Montana State University (top) and USFS Bend Seed Extractory (bottom).
Iltis (1957) described the beeplants as primitive forms in a reduction series which includes the progressively more specialized genera, Cleomella, Wislizenia, and Oxystylis. He suggested the specializations provided adaptation to progressively more arid environments. Characteristics considered primitive in Cleome included their large, elongate capsules; free-falling seeds; short, slender styles; open bracteate racemes; small stipules, extensive vegetative growth, and delayed flowering. The presumed specialized genus, Oxystylis, occurs primarily in arid Death Valley. Later molecular work (see Riser et al. 2013), indicated that Peritoma and Cleomella are polyphyletic, ploidy levels in these genera are complex, fruit size may have diverged from truncated few-seeded fruits of the ancestral Cleomella, and the timing of the evolutionary divergence of these genera does not support the proposed series.
Reproduction
Rocky Mountain beeplant reproduces entirely from seed. Although annual flowering and seed production can vary greatly, plants produce prodigious amounts of seeds in favorable years. Populations can spread rapidly in areas where competition from other species is low (Cane 2008).
Phenology
Rocky Mountain beeplant flowers over a period of several weeks from late spring to mid-summer depending on location. In southern Alberta plants emerge in May, flower buds are produced in June, flowering occurs from June to September, seeds ripen from July to September, and plants die in October (Currah et al. 1983). Flowering begins at the base of the inflorescence and inflorescence branches and proceeds upward as the inflorescence elongates. Thus, older perfect flowers are pollinated, develop capsules, and disperse seed while new flowers continue to develop (Iltis 1958; Munz and Keck 1973; Holmgren and Cronquist 2005). Dehiscence of mature capsules occurs when the two halves (valves) of the capsule separate from the loop-shaped placenta (replum), which remains on the plant and dehisces separately (Fig. 6) (Iltis 1958). Seeds then begin dispersing from the placenta.


Figure 6. Seeds dispersing from the placenta (replum) after the valves of the capsule have fallen away. Photo: Matt Lavin, Montana State University.
Racemes alternate between production of perfect and staminate flowers as has also been noted for C. spinosa (spiny spiderflower) and C. lutea (yellow beeplant) (Murneek 1927; Cane 2008). Staminate flower production increases when racemes are producing large numbers of fruits that require high resource input. Thus, flowering throughout the season alternates between the two flower types, and fruit production occurs over a prolonged period (Cane 2008).
Breeding System
Cane (2008) found Rocky Mountain beeplants were both self-fertile and outcrossing. Fruit set and number of seeds per fruit were examined in flowers of caged plants (pollinators eliminated) manually fertilized with pollen from another flower on the same plant (geitonogamy), plants cross pollinated with pollen from another plant (xenogamy) or allowed to naturally self-fertilize (autogamy). Treatments were compared to non-caged plants with flowers accessible to pollinators. Plants were found to be self-fertile, and this was facilitated by stamens that curl back late in the day after the flowers have opened. However, plants from the two manually fertilized treatments set more fruits (P < 0.05) and their fruits produced more seeds (P < 0.0001) than the naturally self-fertilized (self-or wind-pollinated plants). Neither manual cross-pollination nor open access to pollinators improved fruit or seed production (P > 0.05) compared to the same-plant fertilized treatment.
Self-fertilization is considered a favorable trait for early successional species. Self-fertility insures that seeds will be produced, even in areas where pollinators are scarce or absent on disturbance sites or in years when flowers are abundant and pollinator populations limited (Baker and Stebbins 1965; Lloyd 1992; Cane 2008). Spread of Rocky Mountain beeplant following prolific seed production occurred in common gardens and dense populations have been observed on disturbed sites (Cane 2008).
Pollination
Rocky Mountain beeplant is pollinated by many diurnal bees, wasps, and butterflies that gather nectar and sometimes pollen (Cane 2008). In a study conducted at Logan, Utah, new flowers began opening and nectar drops were visible about 1.5 hours after sunset (7 August), and none opened during the day. By dawn all new flowers had dehisced anthers, receptive stigmas, and large nectar droplets (Cane 2008). Nocturnal flower opening was considered a potentially ancestral trait as no moths or other nocturnal pollinators were noted. Lack of discernable odor at night and bright flower color would suggest adaptation to diurnal pollination (Faegri and van der Pijl 1979; Dafini et al. 1987; Cane 2008).
At the Grand Staircase-Escalante National Monument, Utah, Cleome (= Peritoma) was included among the 16 magnet genera that hosted 75% of all pollinators collected (Carril et al. 2018). Sixty-four generalist species (11 unidentified) were associated with Rocky Mountain beeplant, most of which also pollinated yellow beeplant. Cane (2008) suggested that the scattered populations of the two species are important to generalist pollinators throughout their range but fail to attract specialists due to their fluctuating flower production.
Ecology
Rocky Mountain beeplant is a fast-growing annual that colonizes disturbed sites with medium to coarse-textured soils and may form monocultures (Currah et al. 1983; USDA NRCS 2020). Seedlings are vigorous, and healthy plants are prolific seed producers, but population size and seed production may vary considerably from year to year.
Seed And Seedling Ecology
Competitiveness of native species (including Rocky Mountain beeplant) from the Flaming Gorge National Recreation area in Wyoming with the exotic invasive annual halogeton (Halogeton glomeratus) was examined in a greenhouse study (Prasser and Hild 2016). Ten native species were grown separately (four seedlings per pot) or with two native and two halogeton seedlings per pot for 14 weeks. Seedling survival and growth (leaf number, height, root:shoot, canopy area and specific area) of halogeton was not reduced by the presence of Rocky Mountain beeplant. However, halogeton above-ground biomass (as a percent of total pot biomass) was reduced when grown with either of two annuals (Rocky Mountain beeplant or annual sunflower [Helianthus annuus]) or a perennial grass, sand dropseed [Sporobolus cryptandrus]); which was not the case when halogeton was grown with any of seven other native perennials. Additionally, growth and survival of Rocky Mountain beeplant was unaffected by the presence of halogeton. The authors recommended that native annuals be included in revegetation mixes on sites where halogeton is present.
Wildlife And Livestock Use
Seeds of Rocky Mountain beeplant are used by pocket mice (Perognathus longimembris), mourning doves (Zenaida macroura), and other birds (Martin et al. 1951; Winslow 2014; LBJWC 2019). Seed use by ring–necked pheasants (Phasianus colchicus) is reported from Utah (Martin et al. 1951). Dumroese and Luna (2016) listed the genus Cleome (= Peritoma) as one used by greater sage-grouse (Centrocercus urophasianus) based on their use of golden spiderflower (Peritoma platycarpa) (Pyle 1992). Direct observation of Rocky Mountain beeplant use by greater sage-grouse has not been documented.
Rocky Mountain beeplant is grazed to a limited extent by livestock, but it provides little forage for large mammals and has been described as unpalatable by Hermann (1966). The plant’s foul odor has been suggested as a reason for its limited use by deer (Winslow 2014; LBJWC 2019).
Insects. Rocky Mountain beeplant is a larval host for the checkered white (Pontia protodice) (Xerces Society for Invertebrate Conservation in LBJWC 2019).
Rocky Mountain beeplant populations found along a soil moisture gradient in the dry, short grass prairies of western Nebraska and eastern Colorado were examined for production of methyl glucosinolate and insect damage (Louda et al. 1987). At the moist end of the gradient plants were larger, developed more flowers and seeds, and produced lower concentrations of methy glucosinolate than plants at the drier end of the gradient. Insect predation (species not identified) of seed and herbage were inversely proportional to methyl glucosinolate concentration. Consequently, seed production and the potential for reseeding were greater at the drier end of the gradient (Louda et al. 1987).
Ethnobotany
Rocky Mountain beeplant was an important and widely used food plant for American Indians (Table 1) (White 1945; Coffey 1993). It was one of the most important plants used by the Pueblo people. Because of its many uses, the Hopi and Tewa included it in songs about corn, pumpkins, and cotton, the three main cultivated plants (Castetter 1935; Robbins et al. 1916). Welsh (2015) reported it was a volunteer in fields cultivated by the Pueblo people of the Colorado Plateau from 600 to 1100 AD, and its seeds were found in middens excavated in cliff dwellings from this time period. Rocky Mountain bee plant was also an important food of the Navajo and reportedly a good source of calcium and vitamin A that saved them from starvation on several occasions (Holmgren and Cronquist 2005).
Rocky Mountain beeplant may have been cultivated or occurred as volunteers that reseeded themselves annually (Harrington 1967). Plant parts and seed were harvested and used fresh. Large quantities were also harvested and dried for winter use or for preparation of a dye for pottery. Plants were boiled until thick and black, then dried as cakes that were stored until used for paint or fried for food (Robbins et al. 1916; Harrington 1967). Members of the Cikame society of the Keres and the Zuni Shiwanakwe society are prohibited by religious injunction from eating bee plant before ceremonies (Swank 1932; White 1945).
Table 1. Indian uses of Rocky Mountain beeplant for food.
|
Use |
Preparation |
Tribe/Band |
Source(s) |
|
Bread |
Seeds used as flour |
Isleta |
Jones 1931 Castetter 1935 |
|
Cakes |
Fibrous material removed, green parts boiled, made into fried cakes |
Jemez |
Cook 1930 |
|
Cakes |
Seeds mixed with corn, ground and baked as cakes |
Navajo |
Young 1938 cited in Harrington 1967 |
|
Chile |
Young plants, leaves cooked with corn, chile |
Zuni |
Stevenson 1915 Castetter 1935 |
|
Flower buds |
Eaten with salt |
San Filipe |
Castetter 1935 |
|
Greens, cooked |
Leaves, flowers, or young shoots or plants boiled and eaten or rolled into balls and fried, eaten with or without meat |
Hopi Navajo Sia Tewa Ramah Navajo |
Fewkes 1896 Castetter 1935 Whiting 1939 Castetter 1935 Steggerda 1941 Elmore 1944 White 1962 Castetter 1935 Robbins et al. 1916 Vestal 1952 |
|
Greens, fresh |
Leaves, shoots, or young plants |
Apache Isleta Jemez Keres Keresan Navajo |
Buskirk 1986 Jones 1931 Cook 1930 Swank 1932 White 1945 Steggerda 1941 |
|
Stored for winter |
Leaves gathered and dried indoors |
Navajo Zuni |
Lynch 1986 Stevenson 1915 |
|
Stored for winter |
Young shoots or plants boiled, rolled, dried as balls |
Navajo Ramah Navajo |
Castetter 1935 Steggerda 1941 Elmore 1944 Vestal 1952 |
|
Pods |
Unspecified |
Navajo |
Elmore 1944 |
|
Porridge |
Seeds cooked, dried, prepared as mush |
Acoma Keres Laguna |
Castetter 1935 Swank 1932 Castetter 1935 |
|
Spice |
Unspecified |
Navajo |
Hocking 1956 |
|
Seeds |
Unspecified or cooked |
Havasupai Keresan Sia |
Weber and Seaman 1985 White 1945 White 1962 |
|
Stew |
Leaves dried, stored, used in to stew or dumplings |
Navajo |
Castetter 1935 Elmore 1944 Lynch 1986 |
|
Feed for sheep, horses |
Young plants |
Ramah Navajo |
Vestal 1952 |
Leaves of Rocky Mountain beeplant have been used to treat a variety of medical conditions including insect bites, body odors, inflammation, and intestinal problems, and to prepare for ceremonies (Table 2; Vestal 1952; Spellenberg 2001; Curtin 1947 in Vanderpool and Iltis 2010).
Table 2. Native American medicinal uses of Rocky Mountain beeplant.
|
Use/Treatment |
Preparation |
Tribe/Band |
Source |
|
Body, shoe deodorant |
Cold infusion of leaves placed in shoes or on body |
Ramah Navajo |
Vestal 1952 |
|
Fever |
Infusion of whole plant |
Various Oregon tribes |
Murphey 1990 |
|
“Good blood”, improved voice |
Tea made with seeds used ceremonially by Nightway god-impersonators |
Ramah Navajo |
Vestal 1952 |
|
Sore eyes |
Poultice of pounded, soaked leaves |
Gosiute |
Chamberlin 1911 |
|
Stomach disorders |
Infusion taken, poultice of fresh plants used on abdomen |
Tewa |
Robbins et al. 1916 |
The Hopi and other Pueblo Indians used leaves and stems of Rocky Mountain beeplant to prepare a black paint for baskets and pottery (Table 3). Herbaceous material was harvested in early summer when the iron content is high. Seeds and leaves were used to produce a black dye (Curtain and Moore 1997; Murphy 1990; Coffey 1993; Holmgren and Cronquist 2005). Plants were boiled in water until the mixture became thick and turned black. The mixture was dried as cakes that could be stored and later soaked in hot water to reach a desirable consistency (Coffey 1993; Harrington 1967). The Navajo boiled young plants with alum to make a greenish-yellow dye for wool (Holmgren and Cronquist 2005).
Table 3. Native American preparation and use of Rocky Mountain beeplant as paint or dye.
|
Preparation |
Tribe |
Source |
|
Roots |
Isleta |
Jones 1931 |
|
Unspecified plant parts used to decorate pottery |
Keresan |
White 1945 |
|
Young plants boiled, dried, soaked in hot water to make black paint for pottery |
Tewa |
Robbins et al. 1916 |
|
Paste made from whole plant used to decorate pottery and sticks of plume offerings to anthropic gods |
Zuni |
Stevenson 1915 |
Other historic and current uses. Rocky Mountain beeplant can be used as a potherb. Prolonged boiling and two or three changes of water reduce the alkali taste and can reduce the unpleasant odor of mature plants (Robbins et al. 1916; Harrington 1967). Early in the season basal leaves are used as greens as they lack the strong odor (Steggerda 1941). Spanish-American colonists used the seeds to make tortillas during droughts (Spellenberg 2001).
Rocky Mountain beeplant has been used as a nectar source for domestic honeybees since about 1880 (Holmgren and Cronquist 2005; Bailey 1900–1902 in Vanderpool and Iltis 2010). It is now sometimes planted as a honey plant (Lovell 1968; Kindscher 1987). Lovell (1968) advocated its use by apiarists as it began blooming by early June following spring planting in Troutdale, Oregon, produced thousands of flowers, which attracted many bees, and continued flowering until frosts in November.
Horticulture
Rocky Mountain beeplant is a showy annual that produces colorful flowers and interesting fruits over many weeks from early to late summer. It provides a valuable addition to wildflower gardens and urban green spaces, and as a moderate to low water-use annual, it can be used in xeriscaping. The seeds are used by birds, and the flowers provide nectar for a wide variety of bees, wasps, and butterflies over a long period (Cane 2008), making it an excellent species for pollinator and butterfly gardens in the Great Basin and Intermountain Semidesert regions (Ogle et al. 2012; Cane and Kervin 2013; Fallon et al. 2016, Ley et al. 2019).
Seed is available in small quantities and larger seed lots are occasionally produced or can be grown under contract for major landscaping projects. Rocky Mountain beeplant should be grown on medium-textured to sandy soils with full exposure to sun. The species is not highly competitive and should be planted in open spots in the garden. It is, however, a prolific seeder and will reseed itself and may spread into any adjoining open areas. The plant requires little fertilizer or water. Although Atteberry (2019) described it as disease and pest free, Shock et al. (2019) reported extensive damage by flea beetles (Phylotreta cruciferae) in some years at the Oregon State University Malheur Experiment Station (OSU MES) near Ontario, Oregon. The plant is considered somewhat resistant to wildlife browsing, perhaps because of its disagreeable odor (Winslow 2014).
Revegetation Use
Rocky Mountain beeplant is an early successional annual used in revegetation of disturbed sites to provide some cover and stability in arid and semi-arid areas of the West that receive at least 8 to 10 in (200–250 mm) of annual precipitation (Cane 2008; Ogle et al. 2012; Ley et al. 2019). It is recommended for planting in herbaceous wind barriers, field borders, hedgerow plantings, range plantings, upland game bird habitat, and tree and shrub plantings in the Great Basin, Intermountain Semidesert Province, Arizona, Montana, and Wyoming (Ogle et al. 2012; The Xerces Society and USDA NRCS 2012; Winslow 2014; Fallon et al. 2016; LBJWC 2019; Ley et al. 2019). The flowers of Rocky Mountain beeplant provide a valuable nectar source for numerous bees, wasps, butterflies, including Monarch butterflies (Danaus plexippus). Rocky Mountain beeplant is a particularly valuable low water use species for naturalizing and beautifying low maintenance plantings on roadways, rest areas, and campgrounds. Plants develop rapidly, tolerate drought, and spread from seed when competition from other species is low.
Seeds germinate readily following overwinter stratification and can produce abundant seedlings, flowers, and seeds in favorable precipitation years. Because the flowers are self-pollinating and outcrossing, they can produce viable seed on disturbances where pollinators are rare (Cane 2008). Many of its pollinators are ground-nesting bees that nest deep enough to survive fires. Seeding it in selected areas or rows in post-fire seedings on adapted sites could provide a nectar and pollen source through mid-summer to early fall months for remnant pollinators during the first few post-fire seasons before recovering and seeded forbs begin to flower (Cane 2008).
Seed of Rocky Mountain beeplant is generally commercially available in limited quantities. In the Great Basin, annual sales, primarily to private companies, may average 2,000 to 4,000 lbs (900 to 1,800 kg) (R. Stevens, Utah Division of Wildlife Resources (retired), personal communication, February 2020).
Developing A Seed Supply
For restoration to be successful, the right seed needs to be planted in the right place at the right time. Coordinated planning and cooperation is required among partners to first select appropriate species and seed sources and then properly collect, grow, certify, clean, store, and distribute seed for restoration (PCA 2015).
Developing a seed supply begins with seed collection from native stands. Collection sites are determined by current or projected revegetation requirements and goals. Production of nursery stock requires less seed than large-scale seeding operations, which may require establishment of agricultural seed production fields. Regardless of the size and complexity of any revegetation effort, seed certification is essential for tracking seed origin from collection through use (UCIA 2015).
Seed Sourcing
Because empirical seed zones are not currently available for Rocky Mountain beeplant, generalized provisional seed zones developed by Bower et al. (2014), may be used to select and deploy seed sources. These provisional seed zones identify areas of climatic similarity with comparable winter minimum temperature and aridity (annual heat:moisture index). In Figure 7, Omernik Level III Ecoregions (Omernik 1987) overlay the provisional seeds zones to identify climatically similar but ecologically different areas. For site–specific disturbance regimes and revegetation objectives, seed collection locations within a seed zone and ecoregion may be further limited by elevation, soil type, or other factors. For horticultural use, PFAF (2019) recommends planting the species in USDA Hardiness zones 3–8, although cautioning that the species may be frost tender.
The Western Wildland Environmental Threat Assessment Center’s (USFS WWETAC 2017) Threat and Resource Mapping (TRM) Seed Zone application provides links to interactive mapping features useful for seed collection and deployment planning. The Seedlot Selection Tool (Howe et al. 2017) and the Climate Smart Restoration Tool (Richardson et al. 2019) can also guide revegetation planning, seed collection, and seed deployment, particularly when addressing climate change considerations.
Occurrence Map
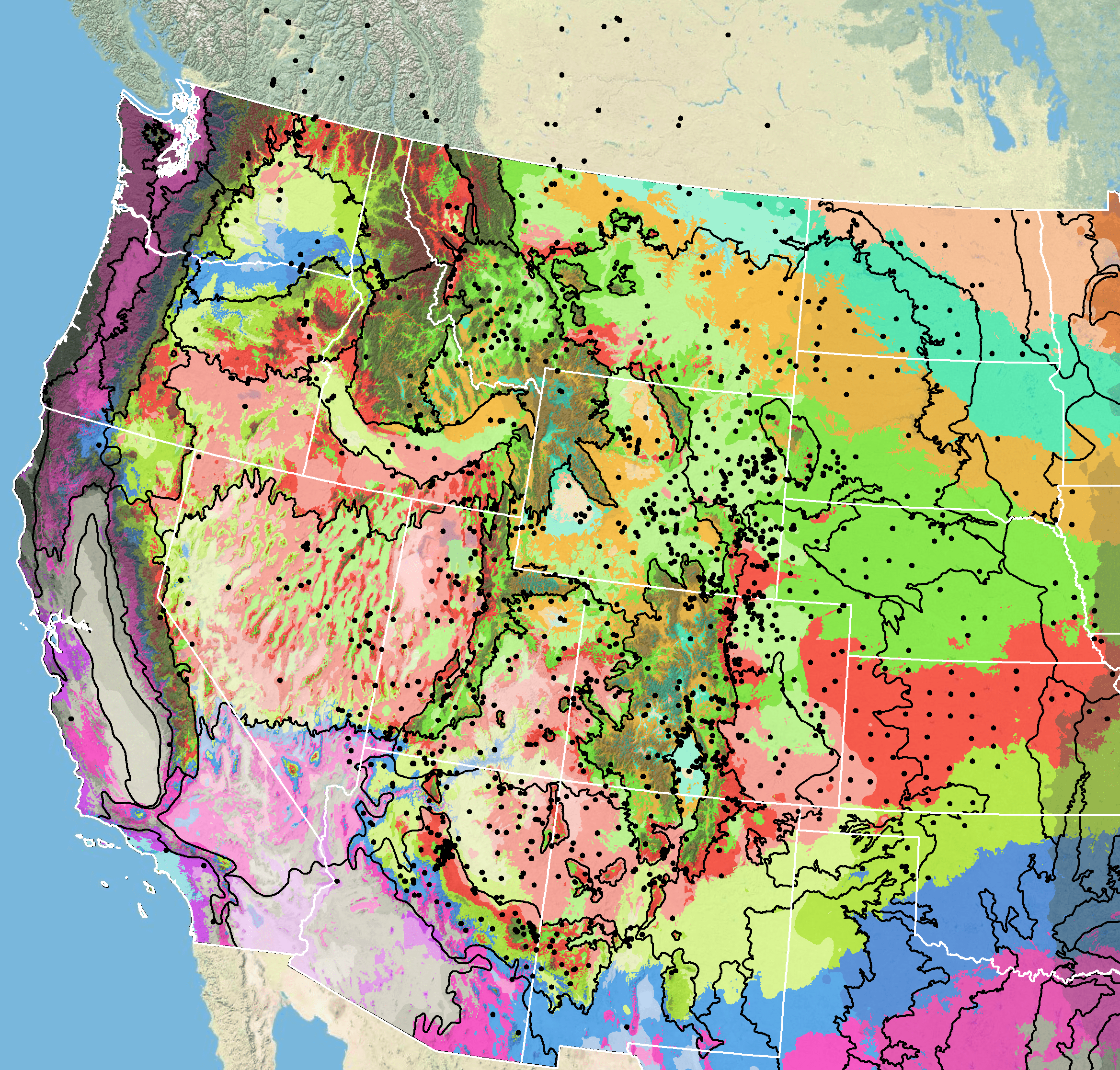
Figure 7. Distribution of Rocky Mountain beeplant (black circles) based on geo-referenced herbarium specimens and observational data from 1874-2015 plus specimens lacking dates (CPNWH 2017; SEINet 2017; USGS 2017). Generalized provisional seed zones (colored regions) (Bower et al. 2014) are overlain by Omernik Level III Ecoregions (black outlines) (Omernik 1987; USDI EPA 2018). Interactive maps, legends, and a mobile app are available (USFS WWETAC 2017; www.fs.fed.us/wwetac/threat-map/TRMSeedZoneMapper2.php?). Map prepared by M. Fisk, USDI USGS.
Releases
As of January 2020, there were no releases of Rocky Mountain beeplant.
Wildland Seed Collection
It is essential to check sites in advance of the expected collection period as seed production and the extent of seed predation by insects is highly variable from year to year. Prodigious crops may be produced in some years, while practically no seed develops in others.
An X–ray or cut test can be used to provide a check of seed quality as seeds begin to ripen. Cane (2008) reported that seeds that were dark colored at harvest or that darkened within 1 week of harvest contained embryos that were viable. Most seeds that remained pale colored generally lacked developed embryos.
Wildland Seed Certification
Verification of species and tracking of geographic source is necessary whether wildland seed is collected for immediate project use or as stock seed for cultivated increase. This official Source Identification process can be accomplished by following procedures established by the Association of Official Seed Certifying Agencies (AOSCA) Pre-Variety Germplasm Program (Young et al. 2020; UCIA 2015). Wildland seed collectors should become acquainted with state certification agency procedures, regulations, and deadlines in the states where they collect.
If wildland–collected seed is to be sold for direct use in ecological restoration projects, collectors must apply for Source-Identified certification prior to making collections. Pre-collection applications, site inspections, and species and seed amount verification are handled by the AOSCA member state agency where seed collections will be made (see listings at AOSCA.org).
If wildland seed collected by a grower or private collector is to be used as stock seed for planting cultivated seed fields or for nursery propagation (See Agricultural Seed Field Certification section), detailed information regarding collection site and collecting procedures must be provided when applying for certification. Photos and herbarium specimens may be required. Germplasm accessions acquired within established protocols of recognized public agencies, however, are normally eligible to enter the certification process as stock seed without routine certification agency site inspections. For contract grow–outs, however, this collection site information must be provided to the grower to enable certification.
Collection Timing
Populations and seed production of this annual can vary considerably from year to year. It is important to monitor flowering and fruit development prior to collection dates in order to identify collectable populations. Seeds are mature when fruit valves begin splitting along the sutures (Winslow 2014). There is generally a wide collection window for harvesting seed as plants ripen indeterminately over a long period from May to October, depending on location. Collections may be made on multiple dates to increase genetic diversity if sites are easily accessible. In addition, and depending upon site topography and conditions, plants in different microsites within an area (depressions, ridges, slopes, etc.) may be at different flowering and fruit ripening stages on any one collection date.
Wildland seed collections made by BLM Seeds of Success (SOS) interns (USDI BLM SOS 2017) were harvested from mid–July to early October, but most collections were made in August or September. Seed was harvested in six states: Arizona, Colorado, Nevada, New Mexico, Utah, and Wyoming at elevations ranging from 4,691 ft (1,430 m) in Juab County, Utah, to 8,074 ft (2,461 m) in Wayne County, Utah. Based on 30 SOS collections made over 9 years, the earliest collection date was July 15 in Kane County, Utah, at 5,757 ft (1,755 m) in the Colorado Plateau ecoregion (Omernik 1987) and the latest was October 10 in Millard County, Utah, at 4,761 ft (1,451 m) in the Central Basin and Range Ecoregion (USDI BLM SOS 2017).
Collection Methods
Fruit ripening is highly indeterminate with fruit at the base of the raceme ripening earliest. At maturity, the two valves of mature capsules begin separating from the loop-like placenta (replum). Seeds are attached to the placenta and soon begin dispersing. Collectable fruits are those that dehisce readily or that have begun to dehisce along the valves. These are readily hand stripped with minimal disturbance to immature fruits further up the raceme. Fruits can be stripped by hand or using a flat beater (e.g., tennis racket) into bags, boxes, or a collection hopper. Thin gloves may be worn to protect the hands from abrasion. Collections will consist of a mixture of seeds, valves, and fine debris (Fig. 8). Some commercial collectors harvest seed mechanically in larger stands using a sweeping type seed harvester when the lower pods are ripe. Green pods on the inflorescences must be dried and some additional seed will mature during drying (R. Stevens, Utah Division of Wildlife Resources, personal communication, February 2020).
Several collection guidelines and methods should be followed to maximize the genetic diversity of wildland collections: 1) collect seed from a minimum of 50 randomly selected plants; 2) collect from widely separated individuals throughout a population without favoring the most robust or avoiding small stature plants; and 3) collect from all microsites including habitat edges (Basey et al. 2015). General collecting recommendations and guidelines are provided in online manuals (e.g., ENSCONET 2009; USDI BLM SOS 2021).
It is critical that wildland seed collection does not impact the sustainability of native plant populations. Collectors should take no more than 20% of the viable seed available at the time of harvest (USDI BLM SOS 2021). Additionally, care must be taken to avoid the inadvertent collection of weedy species, particularly those that produce seeds similar in shape and size to those of Rocky Mountain beeplant.
Post-Collection Management
Collections should be dried thoroughly as some capsules and vegetative material may not be completely dry at the time of harvest. The seed lot should be spread over screens in a thin layer to provide for good air circulation. Seeds should be dried in a location free of rodents and treated to control insects as necessary with the use of appropriate chemicals or by freezing once the seed is dry.
Seed Cleaning
Dried seed can be cleaned using a debearder and an air screen separator (Stevens et al. 1996). At the USFS Bend Seed Extractory, large stems are removed from Rocky Mountain beeplant seed lots by hand (K. Herriman, USFS Bend Seed Extractory, personal communication, January 2020). Seed is then sieved using soil sieve screens, usually numbers 4, 5, or 6, to remove the pod valves and other coarse material. A Westrup LA–P deawner (Westrup A/S, Slagelse, Denmark) is then used to break up remaining pod material and release any seed that remains in the pods. The seed lot is then sieved again to remove free seeds. If any seed remains in the pod material at that point, another cycle of deawning and sieving is conducted. After each step, freed seeds are removed to reduce the risk of mechanical damage by repeated abrasion as well as the quantity of material yet to be cleaned.
The remaining material is put on a finishing machine. Larger amounts are conditioned on an Office Clipper with a round screen (size 6 to 12 as appropriate) on top to remove stems and pod material and the air is set at 1 to 5 to separate smaller pod pieces and empty seed. If the amount of material is small, the seed is put on a continuous seed blower (CSB) (Mater Seed Equipment, Corvallis, OR) to separate filled seed from the empty seed and remaining pod material. Air is set from 300 to 400 depending on seed size (K. Herriman, USFS Bend Seed Extractory, personal communication, January 2020).

Figure 8. Harvested Rocky Mountain beeplant prior to cleaning consists of capsule valves, seed, and fine debris. Photo: USFS Provo Shrub Sciences Laboratory SOS.
Seed Storage
Seeds are orthodox and should be stored dry. Seed longevity in dry storage is 5 or more years (Stevens et al. 1996).
Seed Testing
There is no AOSA rule for testing the germination of Peritoma species (AOSA 2016). Purity tests can be conducted using standard procedures. Viability is tested as for other Cleomaceae (AOSA 2010). A thin slice is cut along the widest dimension of imbibed seed. Seeds are then stained in 0.1% tetrazolium chloride (TZ) overnight. Viable normal embryos are those that stain evenly; slight damage to the radicle is acceptable.
Viability Testing
Cane (2008) found that only dark-colored seed and seed that turned dark within 1 week of harvest contained viable embryos and were readily germinable following cool stratification. Light-colored seeds with pale embryos were generally nonviable and lacked fully developed embryos.
Germination Biology
Little information has been published on the germination requirements of Rocky Mountain beeplant. Laboratory prechilling or fall planting to provide overwinter exposure to prechilling temperatures is required to relieve seed dormancy and enhance germination (Currah 1983; J. Cane, USDA ARS (retired), personal communication, March 2019). Stevens et al. (1996) recommended a 2- to 6-week stratification period and noted that maximum germination is not obtained until 2- or 3-months post-harvest. Tom Clothier (2019) noted that oscillating temperatures are required for germination. Seed can be direct sown in fall or sown in a greenhouse at 80 °F (27 °C) day/70 °F (21 °C) night with germination occurring at 10 to 20 days (Stevens et al. 1996). Prasser and Hild (2016) obtained germination in an 18-day period by incubating seeds at alternating 12 hr day/12 hr night temperatures of 68 °F (20 °C)/50°F (10 °C). Bird in PAFF (2019) reported that germination occurred in a greenhouse at 68 °F (25 °C) in 5 to 14 days.
Wildland Seed Yield And Quality
Cane (2008) reported that three openly pollinated plants in a Logan, Utah, common garden produced more than 20,000 seeds, each with seed weight of 68,040/lb [150,000/kg]. Shock et al. (2017a) found weight of seeds produced in research plots at the OSU MES was 60,800/lb (134,040/kg) at 90% viability. Protein content of Rocky Mountain beeplant seeds has been reported as 18.1% and oil content 23.7% (Earle and Jones 1962).
Post-cleaning seed yield and quality of seed lots collected in the Intermountain region are provided in Table 4 (USFS BSE 2017). High variability in clean-out ratios may have resulted from differences in the quantity of pod valves collected in the bulk seed lot. This, in turn, may have varied with collection method or level of seed maturity on the collection date(s). The results indicate that cleaned seed lots of Rocky Mountain beeplant are nearly pure seed and that fill and viability of fresh seed are often quite high. The number of seeds per bulk pound of cleaned seed and the number of PLS seeds per pound varied considerably among seed lots (Table 4; USFS BSE 2017). Other reports of seed weight (58,961/lb to 90,800/lb [129,987/kg–200,180]) also fall into this range (Earle and Jones 1962; Stevens 1932; Swingle 1939; Currah et al. 1983; USFS GBNPP 2014; RBG Kew 2017; USDA NRCS 2020), except for one report of 174,600 seeds/lb (384,927/kg) (Earle and Jones 1962).
Table 4. Seed yield and quality of Rocky Mountain beeplant seed lots collected in the Intermountain region, cleaned by the Bend Seed Extractory, and tested by the Oregon State Seed Laboratory or the USFS National Seed Laboratory (USFS BSE 2017).
|
Seed lot characteristic |
Mean |
Range |
Samples (no.) |
|
Bulk weight (lbs) |
4.38 |
0.18–14.95 |
29 |
|
Clean weight (lbs) |
2.13 |
0.12–10.27 |
29 |
|
Clean–out ratio |
0.53 |
0.20–0.95 |
29 |
|
Purity (%) |
98 |
93–99 |
29 |
|
Fill (%)¹ |
94 |
86–99 |
29 |
|
Viability (%)² |
94 |
81–98 |
29 |
|
Seeds/lb |
72,239 |
52,005–92,006 |
29 |
|
Pure live seeds/lb |
67,078 |
49,889–81,977 |
29 |
¹ 100 seed X–ray test
² Tetrazolium chloride test
Agricultural Seed Production
Rocky Mountain beeplant is commercially increased in agricultural fields for revegetation use. Much of the research to develop cultural practices for seed production of the species was conducted at the Oregon State University, Malheur Experiment Station (OSU MES) near Ontario, Oregon.
Agricultural Seed Certification
In order to minimize genetic changes in specific accessions of native species when increased in cultivated fields, it is essential to track the geographic source and prevent inadvertent hybridization or selection pressure. This is accomplished by following third party seed certification protocols for Pre-Variety Germplasm (PVG) as established by the Association of Official Seed Certification Agencies (AOSCA). AOSCA members in the U.S., Canada, and other countries administer PVG requirements and standards that track the source and generation of planting stock. Field and cleaning facility inspections then monitor stand establishment, proper isolation distances, control of prohibited weeds, seed harvesting, cleaning, sampling, testing, and labeling for commercial sales (Young et al. 2020; UCIA 2015).
Seed growers apply for certification of their production fields prior to planting and plant only certified stock seed of an allowed generation (usually less than four). The systematic and sequential tracking through the certification process requires preplanning, knowing state regulations and deadlines, and is most smoothly navigated by working closely with state certification agency personnel. See the Wildland Seed Certification section for more information on stock seed sourcing.
Site Preparation
Seed should be planted on well-drained, clay loam to gravelly loam soils fallowed to control weeds (Stevens et al. 1996). Seed should be planted at depths of 0.1 to 0.5 in (2.5–12.7 mm) in late fall using a grain drill or single–row seeder. Recommended seeding rates are 20 or 25 PLS/linear ft (66–82 PLS/linear m) at 24- or 36-in (0.6–0.9 m) row spacings, depending on the equipment used for planting and cultivation for weed control (Stevens et al. 1996; Winslow 2014). Ogle et al. (2012) recommended a pure stand seeding rate of 15 lbs/ac (16.8 kg/ha).
At the OSU MES combinations of seed pretreatments and seeding systems were tested to improve stand density of Rocky Mountain beeplant and other Great Basin forbs with problematic establishment (Shock et al. 2017a). Treatments included coating seeds with fungicides; covering seeded plots with row cover, sawdust or sand; and combinations of these treatments. Seeds were manually planted in November 2016. Across all seven treatments, stand was less than 10% of viable seeds planted.
Weed Management
There are no herbicides registered for use on Rocky Mountain beeplant. Manual and mechanical methods are used for weed control (Winslow 2014; Shock et al. 2017b, 2018, 2019). Adequate between-row spacing should be used to permit mechanical cultivation for weed control (Winslow 2014).
Establishment And Growth
Stands may be erratic, even with fall planting (Shock et al. 2019). Seedlings grow rapidly and may begin flowering when only a few inches tall. Seedling vigor is good and most healthy plants mature and set seed (Winslow 2014). Flowering and seed production extend over a long period, and inflorescences continue to elongate during the flowering period (Munz and Keck 1973; Cane 2008; Vanderpool and Iltis 2010; Welsh et al. 2015).
Irrigation
Irrigating as needed to insure seedling establishment and additional irrigation from flowering though seed development to provide a total of 8 to 10 in (200–250 mm) of water was recommended by Stevens et al. (1996).
At OSU MES irrigation research plots of Rocky Mountain beeplant were seeded annually in mid–November from 2010 to 2018 (Fig. 9; Shock et al. 2017b, 2018, 2019). Seeds were planted in strips with four rows 30 in (76 cm) apart on Nyssa silt loam (pH 8.3, 1.1% organic matter) using a small-plot grain drill with disc openers. Seed was planted on the soil surface at the rate of 20 to 30 seeds/linear ft (66–98/linear meter). Sawdust was applied in a narrow band over each seed row at a rate of 0.26 oz/ft of row (24 g/m).
Beds were then covered with N-sulate row cover (N-sulate, DeWitt Co., Inc., Sikeston, MO) using a mechanical plastic mulch layer. The row cover was removed in April following seedling emergence. Beginning in 2013, netting was placed over the plots to prevent predation by birds (Shock et al. 2017b). Subsurface drip irrigation was used to minimize surface wetting, weed establishment, and the risk of damage resulting from infection by fungal pathogens. Established plots received 0, 4 or 8 in (0, 100, or 200 mm) of subsurface drip irrigation in four equal applications about 2 weeks apart between floral bud formation and seed set (Table 5). In 2014, three additional irrigations were applied beginning on August 12 to determine whether the flowering and seed production period could be extended.

 Figure 9. Rocky Mountain beeplant irrigation research plots at the Oregon State University Malheur Experiment Station, Ontario, Oregon. Photo: USFS.
Figure 9. Rocky Mountain beeplant irrigation research plots at the Oregon State University Malheur Experiment Station, Ontario, Oregon. Photo: USFS.
In 2011 seed yield increased with irrigation up to 8 in (200 mm) (Table 6), but there was no response to irrigation from 2012 to 2018. Accumulated degree-days were lower than average in 2011, but above average from 2012–2018, suggesting that the lower temperatures favored continued flowering and seed set (Shock et al. 2019).
The 2013 stand was lost due to flea beetle damage, and yield was very low in 2016, possibly due to a flea beetle infestation that was not controlled by three insecticide applications (Shock et al. 2019). Pod predation by birds may also have contributed to the low yields. Flowering was resumed and extended by additional irrigations in 2014, but seed set late in the season had not matured by the mid-October harvest. Seed set in September 2015 and 2016 also did not mature, but seed set in September 2017 did mature and was harvested.
Table 5. Rocky Mountain beeplant flowering, irrigation, and seed harvest dates at the Oregon State University, Malheur Experiment Station, Ontario, Oregon, 2011 to 2018 (Shock et al. 2019).
|
Year |
Flowering |
Irrigation |
Harvest |
|||
|
Start |
Peak |
End |
Start |
End |
||
|
––––––––––––––––––––––––date––––––––––––––––––––––– |
||||||
|
2011 |
6/25 |
7/30 |
8/15 |
6/21 |
8/2 |
9/26 |
|
2012 |
6/12 |
6/30 |
6/30 |
7/13 |
7/25 |
7/24–8/30 |
|
2013 |
Full stand loss due to flea beetle damage |
|||||
|
2014 |
6/4 |
6/24 |
7/22 |
5/20 |
7/1l |
7/11–7/30 |
|
2015 |
5/20 |
6/24 |
9/15 |
5/20 |
6/30 |
7/1–8/15 |
|
2016 |
5/23 |
–––– |
9/20 |
5/16 |
6/29 |
6/28–8/15 |
|
2017 |
6/7 |
–––– |
9/29 |
6/6 |
9/15 |
7/3–10/4 |
|
2018 |
5/29 |
–––– |
10/1 |
5/30 |
7/5 |
7/16–8/20 |
Table 6. Rocky Mountain beeplant seed yield response (lbs/ac) to irrigation (Shock et al. 2019).
|
Year |
Supplemental irrigation (in/season¹) |
|||
|
0 |
4 |
8 |
LSD (0.05) |
|
|
–––––––––––––––––––lbs/ac–––––––––––––––––––– |
||||
|
2011 |
446.5 |
499.3 |
593.6 |
100.92 |
|
2012 |
184.3 |
162.9 |
194.7 |
NS³ |
|
2013 |
No stand |
|||
|
2014 |
66.3 |
80.0 |
91.3 |
NS |
|
2015 |
54.0 |
41.0 |
37.9 |
NS |
|
2016 |
0.8 |
2.1 |
1.6 |
NS |
|
2017 |
46.5 |
52.3 |
34.8 |
NS |
|
2018 |
0.6 |
0.7 |
0.5 |
NS |
|
Ave |
100.2 |
105.1 |
119.4 |
NS |
¹Irrigation season: Floral bud to seed set.
²LSD (0.10).
³Not significant: No significant trend in seed yield in response to the amount of irrigation.
Pollinator Management
Although Rocky Mountain beeplant flowers are self–fertile, Cane (2008) found that flowers manually pollinated with pollen from a different flower on the same or a different plant and flowers open to insect pollination produced more fruits, each with more seeds than self-pollinated flowers. Because of the massive number of flowers produced, adequate numbers of pollinators would need to be present throughout the flowering period to insure good crops in seed production fields. Managed domestic pollinators of Rocky Mountain beeplant, European honeybees (Apis mellifera) and alfalfa leaf-cutting bees (Megachile rotundata), were recommended for pollinating the species on seed farms. Providing habitat for native ground-nesting bees near seed production fields would benefit Rocky Mountain beeplant. Over time, populations developing in such habitat would also pollinate other native species being grown for seed.
Pest Management
At OSU MES, Shock et al. (2019) found that prompt and sometimes repeated treatment was essential to prevent severe foliage damage or reductions in seed production (Shock et al. 2017a, b; 2018; 2019).
The following fungi utilize Rocky Mountain beeplant as a host: Cercospora cleomes (leaf spot), C. conspicua (Pseudocercospora conspicua), Puccinia aristidae, P. subnitens (Aecidium biforme), and Leveillula spp. (Farr and Rossman 2017). Potential impacts of infection of any of these species in nurseries or seed fields was not reported.
Seed Harvesting
Seeds should be harvested when the capsules begin to open, but before pods dehisce and seeds disperse (Winslow 2014). In Utah, single harvests occurred from about September 15 to 20, and flowers may be present during the harvest period (Stevens et al. 1996). At OSU MES, research plots were harvested manually two to four times each year (Shock et al. 2017b). Tilley et al. (2012) reported that seed of yellow beeplant can be harvested with a vacuum harvester or by swathing and drying the swathed material for a week before harvesting the seed. If this method is followed, careful estimation of the harvest date is necessary to maximize the yield (Winslow 2014). The generally larger and thicker stems of Rocky Mountain beeplant compared to yellow beeplant makes mechanical harvesting more difficult.
Seed Yields And Stand Life
Large plants in an open pollinated common garden in Logan, Utah, were estimated to produce 26,000 seeds per plant, which would amount to 1,800 lbs/ac (2,000 kg/ha) if all seed could be harvested. Due to indeterminate flowering, multiple harvests would be required to capture a large percentage of the seed (Cane 2008). Stevens et al. (1996) estimated that average yields in Utah would range from 200 to 425 lbs/ac (224–476 kg/ha). Annual variation in seed yield results from poor establishment, low seed production, insect infestations, and harvest techniques and dates.
Nursery Practice
Seeds can be surface sown or covered lightly for greenhouse production. Seedlings can be moved to individual pots and planted out in late spring (Bird 1990 in PFAF 2019).
Wildland Seeding And Planting
Rocky Mountain beeplant seed may be most efficiently used if seeded in a weed-free seedbed in patches, alternate rows, or cross planted (Winslow 2014) separately or with other disturbance-adapted species that are not highly competitive. It should be fall seeded by drilling or broadcasting to provide overwinter stratification. Dry soil through the winter may preclude stratification and dormancy release. Pure stand seeding rate is 25 seeds/ft² (269/m²) or 16.8 lbs/ac (18.8 kg/ha) (Ogle et al. 2012). This rate could be used to establish monoculture patches of the species (Winslow 2014). When it is planted with other species, the rate should be adjusted depending on desired final stand composition; rates of 0.25 to 0.5 lbs/ac (0.3–0.6 kg/ha) are recommended. The seeding rate should be doubled for broadcast seeding, and the seed should be covered (Winslow 2014).
Acknowledgements
Funding for Western Forbs: Biology, Ecology, and Use in Restoration was provided by the USDI BLM Great Basin Native Plant Materials Ecoregional Program through the Great Basin Fire Science Exchange. We thank reviewers Ann Hild, University of Wyoming (Retired); Richard Stevens, Utah Division of Wildlife Resources (retired); and Steve Monsen, USFS Rocky Mountain Research Station (retired).
This research was supported in part by the USDA Forest Service, Rocky Mountain Research Station. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA or U.S. Government determination or policy.
Literature Cited
Andersen, B.A.; Holmgren, A.H. 1996. Mountain plants of northeastern Utah. HG 506. Logan, UT: Utah State University. 124 p.
Association of Official Seed Analysts [AOSA]. 2010. AOSA/SCST Tetrazolium testing handbook. Contribution No. 29. Lincoln, NE: Association of Official Seed Analysts.
Association of Official Seed Analysts [AOSA]. 2016. AOSA rules for testing seeds. Vol. 1. Principles and procedures. Washington, DC: Association of Official Seed Analysts.
Atteberry, T. 2019. Cleome serrulata: A Native American addition to the witchs garden and tale, and a friend to bees and butterflies. https://www.gothichorrorstories.com/witches-garden/witch-garden-feature/cleome-serrulata-also-known-as-a-native-american-addition-to-the-witchs-garden-and-to-the-table-as-well/ [Accessed 2020 January 8].
Baker, H.G.; Stebbins, G.L. 1965. The genetics of colonizing species. New York, NY: Academic press. 588 p.
Basey, A.C.; Fant, J.B.; Kramer, A.T. 2015. Producing native plant materials for restoration: 10 rules to collect and maintain genetic diversity. Native Plants Journal. 16(1): 37-53.
Beatley, J.C. 1976. Vascular plants of the Nevada Test Site and central-southern Nevada: Ecologic and geographic distributions. Springfield, VA: U.S. Department of Commerce, Office of Technical Information, Technical Information Center. 308 p.
Blackwell, L.R. 2006. Great Basin wildflowers. Falcon Guides. Guilford, CT: Globe Pequot Press. 281 p.
Bower, A.D.; St. Clair, J.B.; Erickson, V. 2014. Generalized provisional seed zones for native plants. Ecological Applications. 24(5): 913-919.
Buskirk, W. 1986. The Western Apache: Living with the land before 1950. Norman: University of Oklahoma Press. 273 p.
Cane, J.H. 2008. Beeding biologies, seed production and species-rich bee guilds of Cleome lutea and Cleome serrulata (Cleomaceae). Plant Species Biology. 23(3): 152-158.
Cane, J.H.; Kervin, L. 2013. Gardening for native bees in Utah and beyond. ENT-133-09. Logan,UT: Utah State University Cooperative Extension and Utah Plant Pest Diagnostic Laboratory. 13 p.
Carril, O.M.; Griswold, T.; Haefner, J.; Wilson, J.S. 2018. Wild bees of Grand Staircase-Escalante National Monument; richness, abundance, and spatio-temporal beta-diversity. PeerJ 6:e5867. 29 p.
Castetter, E.F. 1935. Ethnobiological studies in the American Southwest. I. Uncultivated native plants used as sources of food. University of New Mexico Bulletin. 4(1): 1-44.
Chamberlin, R.V. 1911. The ethno-botany of the Gosiute Indians of Utah, Memoirs of the American Anthropological Association. 2(5): 331-405.|
Coffey. T. 1993. The history and folklore of North American wild flowers. New York, NY: Facts on File. 356 p.
Consortium of Pacific Northwest Herbaria [CPNWH]. 2017. Seattle, WA: University of Washington Herbarium, Burke Museum of Natural History and Culture. http://www.pnwherbaria.org/index.php
Cook, S.L. 1930. The ethnobotany of Jemez Indians. Albuquerque: University of New Mexico. Thesis. 30 p.
Correll, D. S.; Johnston, M.C. 1979. Manual of the vascular plants of Texas. Richardson, TX: University of Texas. 1881 p.
Craighead, J.J.; Craighead, F.C. Jr.; Davis, R.J. 1963. A field guide to Rocky Mountain wildflowers from northern Arizona and New Mexico to British Columbia. Boston, MA: Houghton Mifflin Company. 277 p.
Currah, R.; Smrecin, A.; Van Dyk, M. 1983. Prairie wildflowers. Edmonton: University of Alberta, Friends of the Devonian Botanic Garden. 300 p.
Curtin, L.S.M.; Moore, M. 1997. Healing herbs of the Upper Rio Grande: Traditional medicine of the Southwest. Santa Fe, NM: Western Edge Press. 236 p.
Dafni, A.; Eisikowitch, D.; Ivri, Y. 1987. Nectar flow and pollinators efficiency in two co-occurring species of Capparis (Capparaceae) in Israel. Plant Systematics and Evolution. 157(3-4): 181-186.
Dumroese, R.K.; Luna, T.; Pinto, J.R.; Landis, T.D. 2016. Forbs: Foundation for restoration of monarch butterflies, other pollinators, and greater sage-grouse in the western United States. Native Plants Journal. 36(4): 499-511.
Earle, F.R; Jones, Q. 1962. Analyses of seed samples from 113 plant families. Economic Botany. 16(4): 221-250.
Elmore, F.H. 1944. Ethnobotany of the Navajo: A monograph of the University of New Mexico and the School of American Research No. 8. Santa Fe, NM: University of New Mexico. 136 p.
European Native Seed Conservation Network [ENSCONET]. 2009. ENSCONET seed collecting manual for wild species. Edition 1: 32 p.
Faegri, K.; van der Pijl, L. 1979. The principles of pollination ecology. New York, NY: Pergamon Press. 304 p.
Fallon, C.; Adamson, N.L.; Jepsen, S.; Sardinas, H.; Stine, A.; Vaughan, M. 2016. Monarch nectar plants: Great Basin. Portland, OR: The Xerces Society for Invertebrate Conservation. 3 p.
Farr, D.F.; Rossman, A.Y. 2017. Fungal databases, U.S. National Fungus Collections. U.S. Department of Agriculture, Agricultural Research Service. https://nt.ars-grin.gov/fungaldatabases/.
Fewkes, J.W. 1896. A contribution to ethnobotany. American Anthropologist. 9(1): 14-21.
Hall, J.C. 2008. Systematics of Capparaceae and Cleomaceae: An evaluation of the generic delimitations of Capparis and Cleome using plastid DNA sequence data. Botany. 86(7): 682-696.
Harrington, H.D. 1967. Edible native plants of the Rocky Mountains. Albuquerque, NM: University of New Mexico Press. 392 p.
Hermann, F. 1966. Notes on western range forbs: Cruciferae through Compositae. Agric. Handb. 293. Washington, DC: U.S. Department of Agriculture, Forest Service. 365 p.
Hocking, G.M. 1956. Some plant materials used medicinally and otherwise by the Navaho Indians in the Chaco Canyon, New Mexico. El Palacio. 63(5-6): 146-165.
Holmgren, P.K.; Cronquist, A. 2005. Cleomaceae, the cleome or spider-flower family. In: Holmgren, N.H.; Holmgren, P.K.; Cronquist, A. Intermountain flora: Vascular plants of the Intermountain West, U.S.A. Volume Two, Part B: Subclass Dilleniidae. Bronx, NY: New York Botanical Garden Press: 160-166.
Howe, G.; St, Clair, B.; Bachelet, D. 2017. Seedlot Selection Tool. Corvallis, OR: Conservation Biology Institute. https://seedlotselectiontool.org/sst/
Iltis, H.H. 1957. Studies in the Capparidaceae. III. Evolution and phylogeny of the western North American Cleomoideae. Annals of the Missouri Botanic Garden. 44(1): 77-119.
Iltis, H.H. 1958. Studies in the Capparidaceae: V. Capparidaceae of New Mexico. The Southwestern Naturalist. 3(1/4): 133-144.
Iltis, H.H.; Hall, J.C.; Cochrane, T.S.; Sytsma, K.J. 2011. Studies in the Cleomaceae I. On the separate recognition of Capparaceae, Cleomaceae, and Brassicaceae. Annals of the Missouri Botanical Garden. 98(1): 28-36.
Jones, V.H. 1931. The Ethnobotany of the Isleta Indians. Albuquerque: University of New Mexico. Thesis. 55 p.
Kindscher, K. 1987. Edible wild plants of the prairie: An ethnobotanical guide. Lawrence: University Press of Kansas. 279 p.
Lady Bird Johnson Wildflower Center [LBJWC]. 2019. Cleome lutea Hook. Native Plant Database. Austin, TX: Lady Bird Johnson Wildflower Center. https://www.wildflower.org/plants-main [Accessed 202 March 21].
Lesica, P.; Lavin, M.; Stickney P.F. 2012. Manual of Montana vascular plants. Fort Worth: Botanical Research Institute of Texas. 779 p.
Ley, E.L.; Buchmann, S.; Stritch, L.; Soltz, G. 2019. Selecting plants for pollinators: A regional guide for farmers, land managers and gardeners in the ecological region of the Intermountain Semidesert Province including states of Washington, Oregon, Idaho, Wyoming and parts of California, Nevada, Utah, Montana, Colorado. San Francisco, CA: The Pollinator Partnership/North American Pollinator Protection Campaign. 23 p. https://www.pollinator.org/PDFs/Intermt.SemiDsrt.Desert.rx2.pdf [Accessed 2019 March 27].
Lloyd, D.G. 1992. Self- and cross-fertilization in plants. II. The selection of self-fertilization. International Journal of Plant Sciences. 153(3): 370-380.
Louda, S.M.; Farris, M.A.; Blua, M.J. 1987. Variation in methylglucosinolate and insect damage to Cleome serrulata (Capparaceae) along a natural soil moisture gradient. Journal of Chemical Ecology. 13(3): 569-581.
Lovell, H.B. 1968. Lets talk about honey plants. Gleanings in Bee Culture. 96(11): 675-676, 697.
Lynch, R.H. 1986. Cookbook. Chinle, AZ: Navajo Curriculum Center, Rough Rock Demonstration School. 44 p.
Martin, A.C.; Zim, H.S.; Nelson, A.L. 1951. American wildlife and plants: A guide to wildlife food habits. New York, NY: Dover Publications. 500 p.
Munz, P.A.; Keck, D.D. 1973. A California flora and supplement. Berkeley, CA: University of California Press. 1905 p.
Murneek, A.E. 1927. Physiology of reproduction in horticultural plants. II. The physiological basis of intermittent sterility with special reference to the spider flower. Columbia, MO: University of Missouri Agricultural Experiment Station Research Bulletin. 106: 1-37.
Murphey, E.V.A. 1990. Indian uses of native plants. Glenwood, IL: Meyerbooks. 81 p.
Nozzolillo, C.; Amiguet, V.T.; Bily, A.C.; Harris, C.S.; Saleem, A.; Anderson, O.M.; Jordheim, M. 2010. Novel aspects of the flowers and flora pigmentation of two Cleome species (Cleomaceae), C. hassleriana and C. serrulata. Biochemical Systematics and Ecology. 38(3): 361-369.
Ogle, D.; St. John, L.; Stannard, M.; Holzworth, L. 2012. Conservation plant species for the Intermountain West. Plant Materials Technical Note 24. Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 57 p.
Omernik, J.M. 1987. Ecoregions of the conterminous United States. Map (scale 1:7,500,000). Annals of the Association of American Geographers. 77(1): 118-125.
Plant Conservation Alliance [PCA]. 2015. National seed strategy for rehabilitation and restoration 2015-2020. Washington, DC: U.S. Department of the Interior, Bureau of Land Management. 52 p.
Plants for a Future [PFAF]. 2019. Cleome serrulata – Pursh. https://pfaf.org/user/Plant.aspx?LatinName=Cleome+serrulata [Accessed 2020 January 2].
Prasser, N.P.; Hild, A.L. 2016. Competitive interactions between an exotic annual, Halogeton glomeratus, and 10 North American native species. Native Plants Journal. 17(3): 244-254.
Pyle, W.H. 1992. Response of brood-rearing habitat of sage grouse to prescribed burning in Oregon. Corvallis, OR: Oregon State University. Thesis. 47 p.
Reveal J.L., Moulton G.E., Schuyler A.E. 1999. The Lewis and Clark collections of vascular plants: names, types, and comments. Proceedings of the Academy of Natural Sciences of Philadelphia. 149: 1-64.
Rice, A.; Glick, L.; Abadi, S.; Einhorn, M.; Kopelman, N.M.; Salman-Minkov, A.; Mayzel, J.; Chay, O.; Mayrose, I. 2015. The Chromosome Counts Database (CCDB) – A community resource of plant chromosome numbers. New Phytologist. 206(1): 19-26.
Richardson, B.; Kilkenny, F.; St. Clair, B.; Stevenson-Molnar, N. 2020. Climate Smart Restoration Tool. https://climaterestorationtool.org/csrt/
Riser, J.P.; Cardinal-McTeague, W.M.; Hall, J.C.; Hahn, W.; Sytsma, K.J.; Roalsen, E.H. 2013. Phyogenetic relationships among the North American cleomoids (Cleomaceae): A test of Iltiss reduction series. American Journal of Botany. 100(10): 2102-2111.
Roalson, E.H.; Hall, J.C.; Riser, J.P.; Cardinal-McTeague, W.M.; Cochrane, T.S.; Sytsma, K.J. 2015. A revision of generic boundaries and nomenclature in the North American cleomoid clade (Cleomaceae). Phytotaxa. 205(3): 129-144.
Robbins, W.W.; Harrington, J.P.; Freire-Marreco, B. 1916. Ethnobotany of the Tewa Indians, SI-BAE Bulletin #55. Washington, DC: Smithsonian Institution, Bureau of American Ethnology. 124 p.
Rogers, D.J. 1980. Edible, medicinal, useful, and poisonous wild plants of the northern Great Plains – South Dakota Region. Sioux Falls, SD: Augustana College. 129 p.
Royal Botanic Gardens Kew [RBG Kew]. 2017. Seed Information Database (SID). Version 7.1. http://data.kew.org/sid/
Sanchez-Acebo, L. 2005. A phylogenetic study of the new world Cleome (Brassicaceae, Cleomoideae). Annals of the Missouri Botanical Garden. 92(2): 179-201.
SEINet – Regional Networks of North American Herbaria Steering Committee [SEINet]. 2017. SEINet Regional Networks of North American Herbaria. https://symbiota.org/seinet
Shock, C.C.; Feibert, E.B.G.; Rivera, A.; Sanders, L.D.; Kilkenny, F.; Shaw, N. 2017a. Direct surface seeding systems for the establishment of native plants in 2016. In: Shock, C.C., ed. Malheur Experiment Station Annual Report 2016. OSU AES Ext/CRS 157. Corvallis, OR: Oregon State University: 123-130.
Shock, C.C.; Feibert, E.B.G.; Rivera, A.; Sanders, L.D.; Shaw, N.; Kilkenny, F. 2017b. Native beeplant seed production in response to irrigation in a semi-arid environment. In: Shock, C.C., ed. Malheur Experiment Station Annual Report 2016. OSU AES Ext/CRS 157. Corvallis, OR: Oregon State University: 140-144.
Shock, C.C.; Feibert, E.B.G.; Rivera, A.; Sanders, L.D.; Shaw, N.; Kilkenny, F. 2018. Native beeplant seed production in response to irrigation in a semi-arid environment. In: Shock, C.C., ed. Malheur Experiment Station Annual Report 2017. OSU AES Ext/CRS 159. Corvallis, OR: Oregon State University: 154-159.
Shock, C.C.; Feibert, E.B.G.; Rivera, A.; Wieland, K.; Shaw, N.; Kilkenny, F. 2019. Native beeplant seed production in response to irrigation in a semi-arid environment. In: Shock, C.C., ed. Malheur Experiment Station Annual Report 2018. OSU AES Ext/CRS 161. Corvallis, OR: Oregon State University. 7 p.
Spellenberg, R. 2001. National Audubon Society field guide to North American wildflowers: Western region, revised edition. New York, NY: Alfred A. Knopf, Inc. 862 p.
Steggerda, M. 1941. Navajo foods and their preparation. Journal of the American Dietetic Association. 17(3): 217-225.
Stevens, O.A. 1932. The number and weight of seeds produced by weeds. American Journal of Botany. 19(9): 784-794.
Stevens, R.J.; Jorgensen, K.R.; Young, A.S.; Monsen, S.B. 1996. Forb and shrub seed production guide for Utah. AG 501. Logan, UT: Utah State University Extension. 52 p.
Stevenson, M.C. 1915. Ethnobotany of the Zuni Indians, SI-BAE Annual Report #30. Washington, DC: Smithsonian Institution, Bureau of American Ethnology. 102 p.
Swank, G.R. 1932. The ethnobotany of the Acoma and Laguna Indians. Albuquerque, NM: University of New Mexico. Thesis. 86 p.
Swingle, C.F. 1939. Seed propagation of trees, shrubs and forbs for conservation planting. SCS-TP-27. Washington, DC: U.S. Department of Agriculture, Soil Conservation Service. 198 p.
Tilley, D.; Cane, J.; St. John, L.; Ogle, D.; Shaw, N. 2012. Plant guide: yellow beeplant (Cleome lutea). Aberdeen, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 3 p.
Tom Clothier Annual/Biennial Seed Germination Database [Tom Clothier]. 2020. Tom Clothiers Garden Walk and Talk. https://tomclothier.hort.net/page05.html [Accessed 2020 January 13].
USDA Forest Service, Bend Seed Extractory [USDA FS BSE]. 2017. Nursery Management Information System Version 4.1.11. Local Source Report 34-Source Received. Bend, OR: U.S. Department of Agriculture, Forest Service, Bend Seed Extractory.
USDA Forest Service, Great Basin Native Plant Project [USFS GBNPP]. 2014. Seed weight table calculations made in-house. Report on file. Boise, ID: U.S. Department of Agriculture, Forest Service, Boise Aquatic Sciences Laboratory. Available: https://www.fs.fed.us/rm/boise/research/shrub/Links/Seedweights.pdf
USDA Forest Service, Western Wildland Environmental Threat Assessment Center [USFS WWETAC]. 2017. TRM Seed Zone Applications. Prineville, OR: U.S. Department of Agriculture, Forest Service, Western Wildland Environmental Threat Assessment Center. https://www.fs.fed.us/wwetac/threat-map/TRMSeedZoneMapper.php
USDA Natural Resources Conservation Service [USDA NRCS]. 2020. The PLANTS Database. Greensboro, NC: U.S. Department of Agriculture, Natural Resources Conservation Service, National Plant Data Team. https://plants.usda.gov/java
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2016. Bureau of Land Management technical protocol for the collection, study, and conservation of seeds from native plant species for Seeds of Success. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program. 37 p.
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2017. Seeds of Success collection data. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program.
USDI Environmental Protection Agency [USDI EPA]. 2017. Ecoregions. Washington, DC: U.S. Department of the Interior, Environmental Protection Agency. https://www.epa.gov/eco-research/ecoregions
USDI Geological Survey [USDI USGS]. 2017. Biodiversity Information Serving Our Nation (BISON). U.S. Geological Survey. https://bison.usgs.gov/#home
Utah Crop Improvement Association [UCIA]. 2015. How to be a seed connoisseur. Logan, UT: UCIA, Utah Department of Agriculture and Food, Utah State University and Utah State Seed Laboratory. 16 p.
Vanderpool, S.S.; Iltis, H.H. 2010. Peritoma de Candolle. In: Flora of North America Editorial Committee, ed. Flora of North America North of Mexico. Volume 7. Magnoliophyta Salicaceae to Brassicaceae. New York, NY: Oxford University Press: 205-208.
Vestal, P.A. 1952. Ethnobotany of the Ramah Navaho. Reports of the Ramah Project: No. 4. Papers of the Peabody Museum of American Archeology and Ethnology. Cambridge, MA: Harvard University. 40(4): 1-94.
Weber, S.A.; Seaman, P.D., eds. 1985. Havasupai habitat: A.F. Whiting’s ethnography of a traditional Indian culture. Tucson, AZ: University of Arizona Press. 288 p.
Weber, W.A. 1976. Rocky Mountain flora. Boulder, CO: Colorado Associated University Press. 479 p.
Welsh, S.L. 2015. Capparaceae A.L. Jussieu Caper family. In: Welsh, S.L.; Atwood, N.D.; Goodrich, S.; Higgins, L.C., eds. A Utah flora. 5th ed. Provo, UT: Brigham Young University: 93-95.
White, L.A. 1945. Notes on the ethnobotany of the Keres. Papers of the Michigan Academy of Arts, Sciences and Letters. 30: 557-568.
White, L.A. 1962. The pueblo of Sia, New Mexico. SI-BAE Bulletin 184. Washington, DC: Smithsonian Institution, Bureau of American Ethnology. 358 p.
Whiting, A.F. 1939. Ethnobotany of the Hopi. Museum of Northern Arizona Bulletin No. 15. Flagstaff, AZ: Museum of Northern Arizona. 128 p.
Winslow, S.R. 2014. Rocky Mountain beeplant Cleome serrulata Pursh: A native annual forb for conservation use in Montana and Wyoming. Plant Materials Tech. Note MT-104. Bridger, MT: U.S. Department of the Interior, Natural Resources Conservation Service. 5 p.
Xerces Society for Invertebrate Conservation; Natural Resources Conservation Service [Xerces Society & USDA NRCS]. 2012. Plants for enhancing pollinator habitat in Arizona. Tech. Note 12-1. Tucson, AZ: U.S. Department of Agriculture, Natural Resources Conservation Service. 15 p.
Young, S.A.; Schrumpf, B.; Amberson, E. 2003. The Association of Official Seed Certifying Agencies (AOSCA) native plant connection. Moline, IL: AOSCA. 9 p. Available: https://seedcert.oregonstate.edu/sites/seedcert.oregonstate.edu/files/pdfs/aoscanativeplantbrochure.pdf
How to Cite
Shaw, Nancy L.; Gucker, Corey L. 2020. Rocky Mountain beeplant (Peritoma [Cleome] serrulata). In: Gucker, C.L.; Shaw, N.L., eds. Western forbs: Biology, ecology, and use in restoration. Reno, NV: Great Basin Fire Science Exchange. Online: https://westernforbs.org/species/rocky-mountain-beeplant-peritoma-serrulata/