Authorship
Gucker, Corey; Shaw, Nancy
Publication Date
April 2019
Nomenclature
Erigeron linearis (Hook.) Piper is commonly referred to as desert yellow fleabane and belongs to the Osteocaulis section, Conyzinae subtribe, and Astereae tribe within the Asteraceae family (Nesom 2008).
Family
Asteraceae – Aster family
Genus
Erigeron
Species
linearis
NRCS Plant Code
ERLI (USDA NRCS 2017).
Subtaxa
There are no desert yellow fleabane subspecies or varieties recognized.
Synonyms
Erigeron peucephyllus A. Gray, Diplopappus linearis Hooker (Nesom 2006; Welsh et al. 2016; Hitchcock and Cronquist 2018).
Common Names
Desert yellow fleabane, desert yellow daisy, linear-leaf daisy, line-leaved fleabane, narrow-leaved fleabane, and yellow daisy (Applegate 1938; Taylor 1992; Ogle et al. 2011; Welsh et al. 2016; Hitchcock and Cronquist 2018).
Chromosome Number
Chromosome numbers are: 2n = 18, 27, 36, 45 (Solbrig et al. 1969; Strother 1972; Nesom 2006; Welsh et al. 2016).
Hybridization
Desert yellow fleabane occasionally hybridizes with scabland fleabane (E. bloomeri) and blue dwarf fleabane (E. elegantulus) (Cronquist 1947; Cronquist et al. 1994).
Distribution
Desert yellow fleabane is sporadically distributed from southern British Columbia east to central Montana and Wyoming, south to Yosemite National Park in California, and west to central Oregon and Washington (Cronquist 1947; USDA NRCS 2017). Populations are especially common in Washington (Cronquist 1947), disjunct in Montana and Wyoming (USDA NRCS 2017), and restricted to the Pilot and Grouse Creek ranges in Box Elder County, Utah (Welsh et al. 2016).
Habitat And Plant Associations
Open rocky plains, foothills in low-to moderate-elevation grasslands, shrublands (Fig. 1), and woodlands (Fig. 2) are common desert yellow fleabane habitat (Cronquist 1947; Mee et al. 2003; Nesom 2006; Pavek et al. 2012; Hitchcock and Cronquist 2018). Common vegetation associates include Idaho fescue (Festuca idahoensis), bluebunch wheatgrass (Pseudoroegneria spicata), sagebrush (Artemisia spp.), rubber rabbitbrush (Ericameria nauseosus), bitterbrush (Purshia spp.), and juniper (Juniperus spp.) (Cronquist 1947; Nicholson et al. 1991; Nesom 2006; Welsh et al. 2016). Less common associates include ponderosa pine (Pinus ponderosa) and Douglas-fir (Pseudotsuga menziesii) (Lloyd et al. 1990; Nicholson et al. 1991).

Figure 1. Desert yellow fleabane growing in a sagebrush grassland in Idaho. Photo: USDI BLM ID931, Seeds of Success (SOS).
In British Columbia, desert yellow fleabane is common in the bluebunch wheatgrass very dry warm subzone between about 2,300 to 3,300 ft (700–1000 m). Scattered shrubs (prairie sagewort [Artemisia frigida], big sagebrush [A. tridentata], and rubber rabbitbrush) are common and ponderosa pine and Douglas-fir are occasional in this zone (Nicholson et al. 1991). Desert yellow fleabane also occurs in interior Douglas–fir forests in British Columbia’s Kamloops region (Lloyd et al. 1990).
Desert yellow fleabane often occurs in the western juniper (J. occidentalis)/big sagebrush/bluebunch wheatgrass vegetation type which ranges from southeastern Washington south to northern California and eastern Idaho (Shiflet 1994) and is frequent in the northern part of the western juniper woodland zone of southern Washington and Oregon (Dealy 1990). In central Oregon, desert yellow fleabane occurs consistently (40–100% constant), but as a low cover (0.1–0.2%) component in the western juniper zone (Driscoll 1964).

Figure 2. Desert yellow fleabane growing adjacent to a juniper community in Oregon. Photo: USDI BLM OR050 SOS.
Desert yellow fleabane is a characteristic forb in sagebrush communities. It is frequent in low sagebrush (A. arbuscula)/bluebunch wheatgrass and low sagebrush/Idaho fescue communities in the High Lava Plains Owyhee Uplands and Blue Mountains of Oregon (Franklin and Dyrness 1973). In big sagebrush and low sagebrush communities in southeastern Oregon, desert yellow fleabane was 50 to 75% constant in low sagebrush/Idaho fescue communities and 10 to 25% constant in big sagebrush/bluebunch wheatgrass communities (Tueller 1962). In eastern Oregon, desert yellow fleabane occurred on one of six Wyoming big sagebrush (Artemisia tridentata subsp. wyomingensis)/Idaho fescue stands evaluated (Doescher et al. 1986). Desert yellow fleabane production was 6 lbs/ac (6.7 kg/ha) in a Wyoming big sagebrush/Idaho fescue stand on silt loam soil from alluvium parent material with 2% organic matter and 7.4 pH. The 15-year average annual precipitation at this site was 10.3 in (262 mm). The stand occurred on a west aspect and had the lowest total herbage production 159 lbs/ac (178 kg/ha) of all stands. All other stands evaluated occurred on north, east, or northeast aspects (Doescher et al. 1986).
In California, desert yellow fleabane is largely transmontane and occurs on rocky slopes from sagebrush to subalpine fir (Abies lasiocarpa) communities from northern California to Tuolomne County (Munz and Keck 1973). Along an elevation gradient from 5,800 to 8,500 ft (1,775–2,575 m) at sites southwest of Bishop, California, desert yellow fleabane cover was 2% at the (2,575 m) site dominated by big sagebrush. It was absent from the lowest elevation site dominated by Mormon tea (Ephedra viridis), and the 7,125-ft (2,175 m) site dominated by antelope bitterbrush (Purshia tridentata) (Loik and Edar 2003).
Elevation
Desert yellow fleabane is most common at elevations of 2,300 to 6,500 ft (700–2,000 m) (Nesom 2006) but it has also been reported at elevations as high as 10,000 ft (3,000 m) in California (Munz and Keck 1973). In Utah, desert yellow fleabane is restricted to Box Elder County, and its elevation ranges from 5,500 to 6,900 ft (1,700–2,100 m) or more (Welsh et al. 2016).
Soils
Dry rocky or medium to coarse-textured soils (Fig. 3) with neutral pH are common in desert yellow fleabane habitats (Cronquist et al. 1994; Mee et al. 2003; Hitchcock and Cronquist 2018). Desert yellow fleabane is common in shallow, poorly developed soils with partially weathered rock fragments (lithosols) in sagebrush ecosystems, especially the western half of the sagebrush steppe and in basaltic areas of the Columbia Basin (Taylor 1992). Desert yellow fleabane dominated an overgrazed plot with an average soil depth of 2.25 in (5.7 cm) in what was likely an antelope bitterbrush/Douglas’ buckwheat (Eriogonum douglasii) potential vegetation type in the Blue Mountains of Oregon (Johnson and Swanson 2005). In central Oregon’s western juniper zone, desert yellow fleabane had high constancy but low cover at sites with sandy loam to loamy coarse or clay loam soils with pH of 6 to 8 (Driscoll 1964).

Figure 3. Desert yellow fleabane growing in a rocky site in Nevada. Photo: USDI BLM NV020 SOS.
Description
Desert yellow fleabane is a perennial from a stout multibranched taproot giving rise to a branched, woody caudex (Cronquist et al. 1994; Nesom 2006). Taproots typically reach a minimum depth of 12 in (30 cm) (Mee et al. 2003). Branches of the caudex are covered in withered but persistent leaf bases, and plants exude a watery juice when injured (Welsh et al. 2016). Plants are strongly tufted, often with many erect stems mostly from 2 to 8 in (5–20 cm) tall (Fig. 4) (Taylor 1992; Cronquist et al. 1994), but mat-forming plants have also been reported (Bates et al. 2007). Stems and leaves are sparsely to densely covered with fine, stiff, appressed hairs. Bases of the stems are hairless or nearly so, slightly enlarged, and straw to purple in color (Cronquist 1947; Munz and Keck 1973). Leaves are mostly basal but can be well distributed along the stems (Cronquist 1947; Nesom 2006). Leaf bases are enlarged and straw to purple in color (Cronquist 1947; Welsh et al. 2016). Hairiness of leaves is also similar to that of the stems with sparse to dense, stiff, fine, and short hairs all appressed in the same ascending direction (Munz and Keck 1973; Cronquist et al. 1994). Leaves are alternate, simple, entire, and linear from 0.4 to 3.5 in (1–9 cm) long and 0.5 to 3 mm wide (Cronquist 1947; Munz and Keck 1973; Nesom 2006). If present, leaves along the stems are reduced (Mee et al. 2003).

Figure 4. Desert yellow fleabane plant growing in a rocky site in Nevada. Photo: USDI BLM NV020, SOS.
Stems generally bear a single yellow flower head but may have up to three. Flowers are usually bright yellow but can be cream to off white in color (Cronquist et al. 1994; Nesom 2006). Flower bases or involucres are 8 to 13 mm wide and 4 to 7 mm tall with bracts that are green to straw colored and villous to finely glandular (Cronquist et al. 1994; Welsh et al. 2016). Ray florets are typically present and are pistillate and fertile. Disc florets are bisexual and fertile (Mee et al. 2003; Nesom 2006; Pavek et al. 2012; Welsh et al. 2016). When present, ray florets number 15 to 45 and are 4 to 11 mm long and 1.3 to 2.5 mm wide (Cronquist 1947; Welsh et al. 2016). Disc florets are 3.5 to 5.3 mm long (Cronquist et al. 1994; Nesom 2006). Fruits are cypselas with two nerves and a double pappus (10–20 inner bristles) and outer scales. Fruits are 2 to 2.3 mm long and can have moderate coverings of short hairs (Cronquist 1947; Cronquist et al. 1994; Nesom 2006; Welsh et al. 2016).
Reproduction
Desert yellow fleabane reproduces by seed. Flowering generally occurs in the spring, but flowers can occur from May to August throughout the species’ range (Munz and Keck 1973; Cronquist et al. 1994; Nesom 2006). Desert yellow fleabane is often recommended as a pollinator species, as it attracts bees and butterflies (Ogle et al. 2011; Eldredge et al. 2013; Dumroese et al. 2016).
Ecology
Desert yellow fleabane is a slow-growing, drought-tolerant, shade-intolerant plant (Mee et al. 2003; Ogle et al. 2011). Disturbance tolerance is variable and may depend on season of disturbance (see Disturbance Ecology section below).
Seed And Seedling Ecology
In a greenhouse experiment comparing seedling growth of nonnative and native perennial forbs, desert yellow fleabane grew slowly. Desert yellow fleabane seed collected from a population in eastern Oregon was kept at 39 °F (4 °C) for 2 weeks, germinated on moist filter paper, and then transferred into pots filled with soil. Growth comparisons were made among plants in control pots and those in pots with thoroughly mixed or concentrated packets of fertilizer (10-10-10 NPK) buried in the top 2 to 6 in (5-15 cm) of soil. After 12 weeks, desert yellow fleabane produced very few roots (<1 g total root mass, <5 km/m³ root length density, and about 300 km/kg specific root length) regardless of fertilization. Researchers considered it a slow-growing species, with low root foraging precision, photosynthetic rates, and low shoot and root biomass production (Drenovsky et al. 2008).
Disturbance Ecology
In several studies, desert yellow fleabane appeared tolerant of early seral conditions, grazing, and fire, but fire tolerance may be lower when plants are actively growing. In a study to evaluate the effects of big sagebrush habitat fragmentation, species composition was compared in islands of sagebrush vegetation in agricultural areas and in lava flows. Sagebrush islands in agricultural areas were embedded in active dryland wheat farms in Rockland Valley, Idaho, and sagebrush islands in lava flows (kipukas) occurred at Craters of the Moon National Monument and Preserve in Idaho. Desert yellow fleabane had significantly greater cover in agriculture islands than in kipukas. Agriculture islands also supported a greater number and abundance of nonnative species than kipukas (Huntly et al. 2011).
Grazing. Two studies suggest that desert yellow fleabane persists on grazed sites. In a study evaluating grazing effects on floristic composition in northern Nevada, desert yellow fleabane density was greater on grazed than on ungrazed sites. The grazed area was heavily fall-grazed by sheep from 1890 to 1920, year-round grazed by sheep from 1920 to 1978, and grazed by cattle in a three–pasture rest rotation from 1978 to the time of the study (early 1980s). Ungrazed sites were protected for 19 years. Although the differences were not significant, there were three desert yellow fleabane plants per 10 m² plot on the grazed and none on the same-size ungrazed plots (Bethlenfalvay and Dakessian 1984). In vegetation and succession studies comparing grazed and ungrazed sides of a fenceline in big sagebrush and low sagebrush communities in southeastern Oregon, desert yellow fleabane was considered an increaser with grazing use (Tueller 1962).
Fire. The responses of desert yellow fleabane to fire ranged from severely impacted to unchanged in the few fire effects studies available. Desert yellow fleabane was severely impacted by fire when pre and post-fire Wyoming big sagebrush/bluebunch wheatgrass plots were compared in southeastern Oregon. Prefire vegetation was evaluated in June 2001 before the burn in August of the same year. The fire eliminated all Wyoming big sagebrush plants and left very few unburned patches within the fire perimeter. Desert yellow fleabane was one of several mat-forming perennial forbs that was eliminated or substantially reduced in cover and density by the fire and slow to re-establish (Bates et al. 2007). In the same general area, desert yellow fleabane was “slightly” reduced by a late September or early October prescribed fire that burned a mid- to late-seral Wyoming big sagebrush/Thurber’s needlegrass (Festuca thurberi)-Idaho fescue community. The prescribed fire killed 92% of the Wyoming big sagebrush, and fire effects were evaluated 2 to 7 years after burning (Bates et al. 2011). Frequency of desert yellow fleabane was relatively unchanged after a moderate to high intensity fall (September 25) prescribed fire in basin big sagebrush (Artemisia tridentata subsp. tridentata)/Idaho fescue-bluebunch wheatgrass vegetation in central Oregon (Sapsis 1990). Frequency was 9% before burning, 7% in the first, and 10% in the second post-fire year. On spring-burned (May 24) plots, there was a trace of desert yellow fleabane before the fire, and it was absent in the first post-fire year (Sapsis 1990).
Wildlife And Livestock Use
Although described as poor forage (Ogle et al. 2011), desert yellow fleabane can be important in the diets of ungulates (Oldemeyer et al. 1983). In western sagebrush-grasslands, desert yellow fleabane is important in the diets of pronghorn (Antilocapra americana), especially in spring and summer (McInnis 1985). At the Sheldon National Wildlife Refuge in northwestern Nevada, desert yellow fleabane made up 2% or more of bites taken by mule deer (Odocoileus hemionus) in 3 of 18 feeding trials (Oldemeyer et al. 1983).
Desert yellow fleabane is utilized by greater sage-grouse (Centrocercus urophasianus) adults (Rhodes et al. 2010), and fleabane species are a food source for chicks (Dumroese et al. 2015). Luna et al. (2018) indicate that greater sage-grouse feed on desert yellow fleabane flowers. When year-round diets of California quail (Lophortyx californica) were evaluated in eastern Washington, fleabane (Erigeron spp.) made up 0.5% of the total volume of 31 crops taken in the spring but was not recovered from crops collected in other seasons (Crispens et al. 1960).
Desert yellow fleabane is attractive to bees and butterflies (Ogle et al. 2011), a host plant for endemic sagebrush checkerspots (Chlosyne acastus sterope), and important to other insects. Immaculate green hairstreaks (Callophrys affinis) were found resting on desert yellow fleabane in the Okanagan grassland in British Columbia (Young and Marks 2009). Metallic wood–boring beetles (Acmaeodera idahoensis) were collected from desert fleabane flowers in Oregon (Westcott and Raschko 2018).
Ethnobotany
Okanagan-Colville Indians used a decoction of whole desert yellow fleabane plants to treat tuberculosis (Turner et al. 1980 cited in Moerman 2003).
Horticulture
Desert yellow fleabane is recommended for water wise landscapes. It is drought tolerant, cold hardy, and produces many small, bright yellow flowers. Plants grow tightly clustered and spread slowly. Flowering can be prolonged by dead heading (Mee et al. 2003).
Revegetation Use
No reports of desert yellow fleabane’s use in revegetation occurred in the available literature. Yet, this species is attractive to bees and butterflies (Ogle et al. 2011) and recommended for use in developing or restoring pollinator habitat (Eldredge et al. 2013; Dumroese et al. 2016). Desert yellow fleabane is a host plant for larvae of the endemic sagebrush checkerspot. In a laboratory study, sagebrush checkerspot larvae collected from rubber rabbitbrush and yellow rabbitbrush (Chrysothamnus viscidiflorus) in central Washington developed into adults when fed desert yellow fleabane (James and Seymour 2018).
Developing A Seed Supply
For restoration to be successful, the right seed needs to be planted in the right place at the right time. Coordinated planning and cooperation is required among partners to first select appropriate species and seed sources and then properly collect, grow, certify, clean, store, and distribute seed for restoration (PCA 2015).
Developing a seed supply begins with seed collection from native stands. Collection sites are determined by current or projected revegetation requirements and goals. Production of nursery stock requires less seed than large-scale seeding operations, which may require establishment of agricultural seed production fields. Regardless of the size and complexity of any revegetation effort, seed certification is essential for tracking seed origin from collection through use (UCIA 2015).
Seed Sourcing
Because empirical seed zones are not currently available for desert yellow fleabane, generalized provisional seed zones developed by Bower et al. (2014), may be used to select and deploy seed sources. These provisional seed zones identify areas of climatic similarity with comparable winter minimum temperature and aridity (annual heat:moisture index). In Figure 5, Omernik Level III Ecoregions (Omernik 1987) overlay the provisional seeds zones to identify climatically similar but ecologically different areas. For site-specific disturbance regimes and restoration objectives, seed collection locations within a seed zone and ecoregion may be further limited by elevation, soil type, or other factors.
The Western Wildland Environmental Threat Assessment Center’s (USFS WWETAC 2017) Threat and Resource Mapping (TRM) Seed Zone application provides links to interactive mapping features useful for seed collection and deployment planning. The Seedlot Selection Tool (Howe et al. 2017) can also guide restoration planning, seed collection, and seed deployment, particularly when addressing climate change considerations.
Occurrence Map
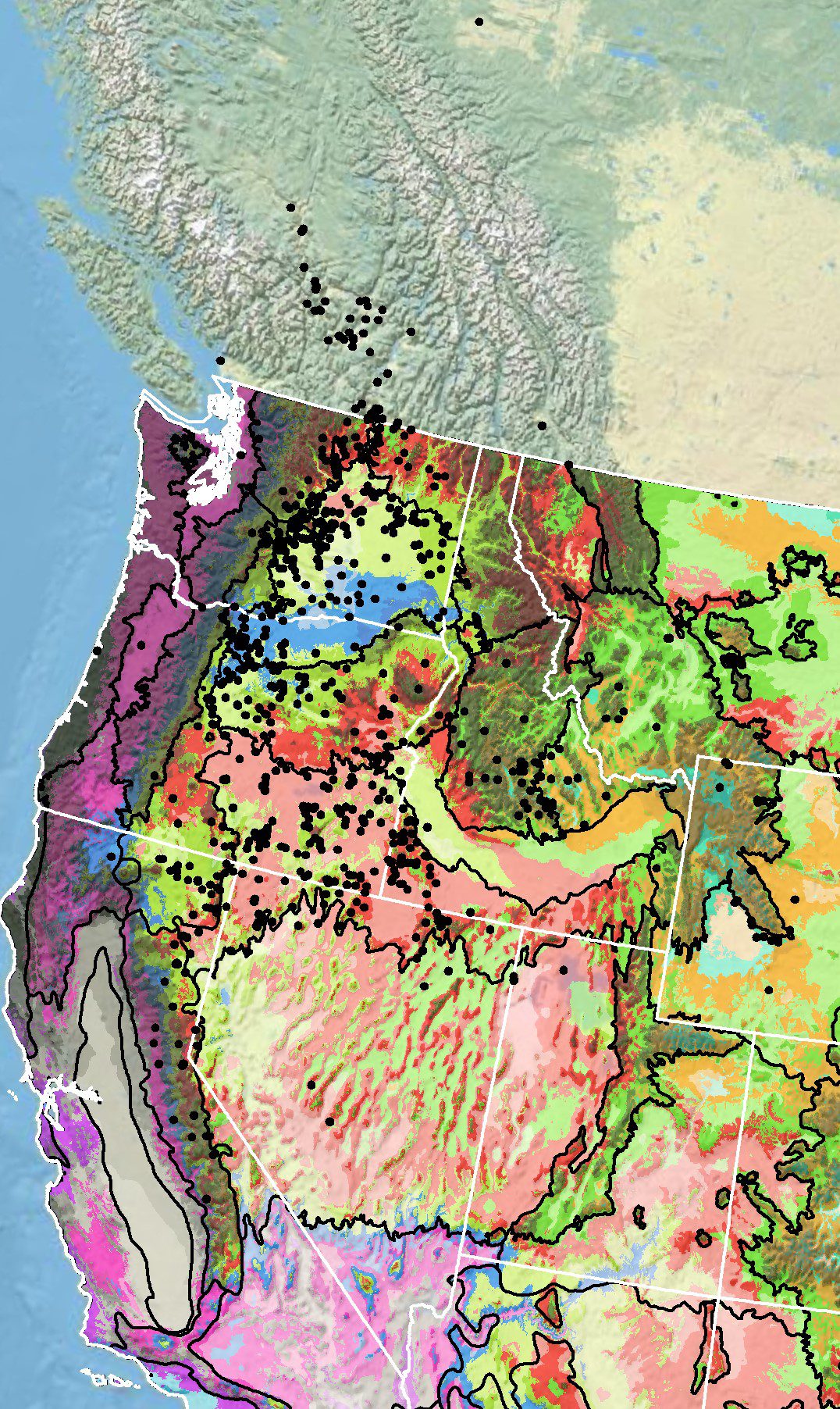
Figure 5. Distribution of desert yellow fleabane (black circles) based on geo-referenced herbarium specimens and observational data from 1885-2011 (CPNWH 2017; SEINet 2017; USDI USGS 2017). Generalized provisional seed zones (colored regions) (Bower et al. 2014) are overlain by Omernik Level III Ecoregions (black outlines) (Omernik 1987; USDI EPA 2018). Interactive maps, legends, and a mobile app are available (USFS WWETAC 2017; www.fs.fed.us/wwetac/threat-map/TRMSeedZoneMapper2.php?). Map prepared by M. Fisk, USDI USGS.
Releases
As of 2019, there were no desert yellow fleabane germplasm releases.
Wildland Seed Collection
Details about wildland seed collection were limited in the literature, though some harvesting clues are provided in plant photographs taken by the USDI BLM Seeds of Success (SOS) field collection crews. Seed appears to be indeterminate (Fig. 6) suggesting that population monitoring and revisiting sites is necessary to maximize harvests.

Figure 6. Indeterminate seed production of a desert yellow fleabane plant in Oregon. Photo: USDI BLM OR030, SOS.
Wildland Seed Certification
Verification of species and tracking of geographic source is necessary whether wildland seed is collected for immediate project use or as stock seed for cultivated increase. This official Source Identification process can be accomplished by following procedures established by the Association of Official Seed Certifying Agencies (AOSCA) Pre-Variety Germplasm Program (Young et al. 2020; UCIA 2015). Wildland seed collectors should become acquainted with state certification agency procedures, regulations, and deadlines in the states where they collect.
If wildland-collected seed is to be sold for direct use in ecological restoration projects, collectors must apply for Source-Identified certification prior to making collections. Pre-collection applications, site inspections, and species and seed amount verification are handled by the AOSCA member state agency where seed collections will be made (see listings at AOSCA.org).
If wildland seed collected by a grower or private collector is to be used as stock seed for planting cultivated seed fields or for nursery propagation (See Agricultural Seed Field Certification section), detailed information regarding collection site and collecting procedures must be provided when applying for certification. Photos and herbarium specimens may be required. Germplasm accessions acquired within established protocols of recognized public agencies, however, are normally eligible to enter the certification process as stock seed without routine certification agency site inspections. For contract grow-outs, however, this collection site information must be provided to the grower to enable certification.
Collection Timing
Data from seed harvests made by the USDI BLM Seeds of Success (SOS) Program suggests that seed is ripe earlier at low than at high elevations (USDI BLM SOS 2017). From 9 years (2003–2016) of SOS seed collections (n = 24) the majority of which were made in Washington, Oregon, Idaho, Nevada, and California, most harvests were made in June (n = 12) or July (n = 10). The earliest harvest occurred on May 28, 2015 in Lincoln County, Washington (elevation not reported). The latest collection date was July 31, 2013 at 4,600 ft (1,400 m) in Crook County, Oregon. In the single year with the most collections (2016), the earliest harvest was May 23 at 2,073 ft (632 m) in Douglas County, Washington, and the latest collections were made on July 7, 2016 at 6,340 ft (1,930 m) in Humboldt County, Nevada, and 6,121 ft (1,866 m) in Lassen County, California (USDI BLM SOS 2017).
Collection Methods
Wildland desert yellow fleabane seed is likely harvested by hand stripping or plucking the seed heads (Fig. 7 and 8).
Several collection guidelines and methods should be followed to maximize the genetic diversity of wildland collections: 1) collect seed from a minimum of 50 randomly selected plants; 2) collect from widely separated individuals throughout a population without favoring the most robust or avoiding small stature plants; and 3) collect from all microsites including habitat edges (Basey et al. 2015). General collecting recommendations and guidelines are provided in online manuals (e.g., ENSCONET 2009; USDI BLM SOS 2021).
It is critical that wildland seed collection does not impact the sustainability of native plant populations. Collectors should take no more than 20% of the viable seed available at the time of harvest (USDI BLM SOS 2021). Additionally, care must be taken to avoid the inadvertent collection of weedy species, particularly those that produce seeds similar in shape and size to those of desert yellow fleabane.

Figure 7. Desert yellow fleabane inflorescences with and without ripe seed. Photo: USDI BLM ID931, SOS.

Figure 8. Desert yellow fleabane bulk seed collection. Photo: USDI BLM OR130, SOS.
Post-Collection Management
Although not specifically discussed in the literature, post-collection management of desert yellow fleabane seed likely requires attention similar to that of most wildland seed. Seed should be dried thoroughly before storing and if insects are suspected, insect strips can be used or seed can be stored at low temperatures to prevent substantial loss to insect damage.
Seed Cleaning
Staff at the Plant Materials Center in Bridger, Montana, developed a seed cleaning method for fleabane seed, when seed was not adequately threshed using a hammermill and manual processing on a rubbing board was too labor intensive and time consuming (Scianna 2004). They used a household blender to clean small quantities (<10 g) of fleabane seed. Blades of the blender were wrapped in duct tape to protect the seeds from damage. If necessary, rice hulls or other inert material was added to the seed to fill the blender to 25 to 33% capacity. Seed was then pulsed at a low speed (Scianna 2004).
Seed cleaning procedures used by the Bend Seed Extractory for lots less than 5 lbs (0.45 kg) were as follows (K. Harriman, U.S. Forest Service, personal communication, April 2019): Desert yellow fleabane seed is sieved to remove non-seed plant material. For very small seed lots, the pappus can be removed by hand rubbing and for larger seed lots, the pappus is removed using the Missoula de-winger. The seed lot is then sieved again to remove any material smaller than the seeds and then processed through a continuous seed blower. If inert material remains that differs in shape or size of the seed, the lot would then be processed through a clipper and sieved again if necessary (K. Harriman, U.S. Forest Service, personal communication, April 2019).
Seed Storage
Desert yellow fleabane seed is orthodox. After 47 days of storage at 15% relative humidity and -4 °F (-20 °C), seeds remained 100% viable (Fig. 9) (RGB Kew 2017).
Seed Testing
Viability is tested using the general procedures described for other Asteraceae genera as no specific AOSA procedure exists for desert yellow fleabane. There is no AOSA rule for testing germination of desert yellow fleabane, but there is one for germination of aspen fleabane (Erigeron speciosus) seed, which may provide a good starting point. The amount of seed needed for purity testing is also provided for aspen fleabane (AOSA 2016).
Germination Biology
There were no germination studies for desert yellow fleabane, but reports suggest that fleabane species require light to germinate (Young and Young 1986) and will germinate at 68 °F (20 °C) (Baskin and Baskin cited in Dumroese et al. 2015).
Wildland Seed Yield And Quality
Post–cleaning seed yield and quality of seed lots collected in the Intermountain region are provided in Table 1 (USFS BSE 2017). The results indicate that desert yellow fleabane seed is tiny. Seed can generally be cleaned to high levels of purity and seed fill and viability of fresh seed is generally high. Others report similar numbers of seeds per pound: 964,894 (USFS GBNPP 2014) and 945,610 (RGB Kew 2017).
Table 1. Seed yield and quality of desert yellow fleabane seed lots collected in the Intermountain region, cleaned by the Bend Seed Extractory, and tested by the Oregon State Seed Laboratory or the USFS National Seed Laboratory (USFS BSE 2017).
|
Seed lot characteristic |
Mean |
Range |
Samples (no.) |
|
Bulk weight (lbs) |
0.19 |
0.05–0.72 |
21 |
|
Clean weight (lbs) |
0.04 |
0.006–0.14 |
21 |
|
Clean–out ratio |
0.19 |
0.017–0.47 |
21 |
|
Purity (%) |
93 |
85–99 |
21 |
|
Fill (%)¹ |
91 |
79–98 |
21 |
|
Viability (%)² |
89 |
69–97 |
18 |
|
Seeds/lb |
1,292,289 |
821,739–1,829,032 |
21 |
|
Pure live seeds/lb |
1,091,264 |
928,546–1,419,329 |
18 |
¹100 seed X–ray test
² Tetrazolium chloride test

Figure 9. Desert yellow fleabane seed collected from plants in Idaho. Photo: USDI BLM ID931, SOS.
Marketing Standards
Acceptable seed purity, viability, and germination specifications vary with revegetation plans. Purity needs are highest for precision seeding equipment used in nurseries, while some rangeland seeding equipment handles less clean seed quite well.
Agricultural Seed Production
Although cultural practices for producing seed A conservation planting guide for establishing pollinator habitat reports that desert yellow fleabane requires 6 to 16 in (152–406 mm) of annual precipitation. It should be seeded at a rate of 4 PLS lbs/ac (4.5 kg/ha), and seedlings develop slowly (Ogle et al. 2011). There were no reports of agricultural seed production of desert yellow fleabane seed.
Desert yellow fleabane is utilized by several pests. Larval and adult western flower thrips (Frankliniella occidentalis) occurred on desert yellow fleabane plants at sites in Washington and Oregon that were adjacent to orchards. A thrip predator (Orius spp.) was also found on desert yellow fleabane plants. The maximum density of western flower thrips was 4.3 adults and larvae pre gram of dry plant material, while the maximum density was 0.4 thrip predators pre gram of dry plant material (Miliczky and Horton 2011). Rust (Puccinia stipae) was collected from desert yellow fleabane in Canada (Farr and Rossman 2017).
Agricultural Seed Certification
In order to minimize genetic changes in specific accessions of native species when increased in cultivated fields, it is essential to track the geographic source and prevent inadvertent hybridization or selection pressure. This is accomplished by following third party seed certification protocols for Pre-Variety Germplasm (PVG) as established by the Association of Official Seed Certification Agencies (AOSCA). AOSCA members in the U.S., Canada, and other countries administer PVG requirements and standards that track the source and generation of planting stock. Field and cleaning facility inspections then monitor stand establishment, proper isolation distances, control of prohibited weeds, seed harvesting, cleaning, sampling, testing, and labeling for commercial sales (Young et al. 2020; UCIA 2015).
Seed growers apply for certification of their production fields prior to planting and plant only certified stock seed of an allowed generation (usually less than four). The systematic and sequential tracking through the certification process requires preplanning, knowing state regulations and deadlines, and is most smoothly navigated by working closely with state certification agency personnel. See the Wildland Seed Certification section for more information on stock seed sourcing.
Nursery Practice
In a greenhouse experiment, researchers described their procedure for growing desert yellow fleabane seedlings (Drenovsky et al. 2008). Seed collected from a population growing in eastern Oregon was stored for 2 weeks at 39 °F (4 °C), germinated on moist filter paper, and the germinants were transferred into pots. Germination was monitored on filter paper because it was low when seeded directly into a 1:1 sand and fritted clay soil mix. Researchers considered desert yellow fleabane a slow-growing species (Drenovsky et al. 2008).
Wildland Seeding And Planting
A conservation planting guide for establishing pollinator habitat reports that desert yellow fleabane is slow growing and adapted to sites with medium to coarse soils, receiving 6 to 16 in (152–406 mm) of annual precipitation. A minimum seeding rate of 4 PLS lbs/ac (4.5 kg/ha) is required, and the recommended seeding depth is 0 to 0.5 in (1.3 cm) (Ogle et al. 2011). Use of desert yellow fleabane in restoration or reclamation sites was not reported in the literature.
Acknowledgements
Funding for Western Forbs: Biology, Ecology, and Use in Restoration was provided by the USDI BLM Great Basin Native Plant Materials Ecoregional Program through the Great Basin Fire Science Exchange. Great thanks to the chapter reviewers: Mary Williams, Nez Perce Tribe and Sarah Barga, USDA USFS RMRS.
This research was supported in part by the USDA Forest Service, Rocky Mountain Research Station. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA or U.S. Government determination or policy.
Literature Cited
Applegate, E.I. 1938. Plants of the Lava Beds National Monument, California. The American Midland Naturalist. 19(2): 334-368.
Association of Official Seed Analysts [AOSA]. 2016. AOSA rules for testing seeds. Vol. 1. Principles and procedures. Washington, DC: Association of Official Seed Analysts.
Basey, A.C.; Fant, J.B.; Kramer, A.T. 2015. Producing native plant materials for restoration: 10 rules to collect and maintain genetic diversity. Native Plants Journal. 16(1): 37-53.
Bates, J.D.; Davies, K.W.; Sharp, R.N. 2007. Response of Wyoming big sagebrush communities to wildfire. In: Davies, K.W.; Nafus, A.M., eds. Sagebrush steppe – Research progress report 2007. Burns, OR: U.S. Department of Agriculture, Agricultural Research Service: 10-21.
Bates, J.D.; Rhodes, E.C.; Davies, K. 2011. Impacts of fire on sage-grouse habitat and diet resources. Natural Resources and Environmental Issues. 17(5): 1-17.
Bethlenfalvay, G.J.; Dakessian, S. 1984. Grazing effects on mycorrhizal colonization and floristic composition of the vegetation on a semiarid range in northern Nevada. Journal of Range Management. 37(4): 312-316.
Bower, A.D.; St. Clair, J.B.; Erickson, V. 2014. Generalized provisional seed zones for native plants. Ecological Applications. 24(5): 913-919.
Consortium of Pacific Northwest Herbaria [CPNWH]. 2017. Seattle, WA: University of Washington Herbarium, Burke Museum of Natural History and Culture. http://www.pnwherbaria.org/index.php
Crispens, C.G.; Buss, I.O.; Yocom, C.F. 1960. Food habits of the California quail in eastern Washington. The Condor. 62(6): 473-477.
Cronquist, A. 1947. Systematic treatment of the species: [Erigeron latus–Erigeron basalticus]. Brittonia 6(2): 192-242.
Cronquist, A.; Holmgren, A.H.; Holmgren, N.H.; Reveal, J.L.; Holmgren, P.K. 1994. Intermountain flora: Vascular plants of the Intermountain West, U.S.A. Volume 5: Asterales. Bronx, NY: The New York Botanic Garden. 496 p.
Dealy, J.E. 1990. Juniperus occidentalis Hook. Western juniper. In: Burns, R.M.; Honkala, B.H., tech cords. Silvics of North America. Volume 1. Conifers. Washington, DC: U.S. Department of Agriculture, Forest Service: 109-115.
Doescher, P.S.; Miller, R.F.; Swanson, S.R.; Winward, A.H. 1986. Identification of the Artemisia tridentata ssp. wyomingensis/Festuca idahoensis habitat type in eastern Oregon. Northwest Science. 60(1): 55-60.
Drenovsky, R.E.; Martin, C.E.; Falasco, M.R.; James, J.J. 2008. Variation in resource acquisition and utilization traits between native and invasive perennial forbs. American Journal of Botany. 95(6): 681-687.
Driscoll, R.S. 1964. Vegetation-soil units in the central Oregon juniper zone. Res. Pap. PNW-19. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 60 p.
Dumroese, R.K.; Luna, T.; Pinto, J.R.; Landis, T.D. 2016. Forbs: Foundation for restoration of monarch butterflies, other pollinators, and greater sage-grouse in the western United States. Native Plants Journal. 36(4): 499-511.
Dumroese, R.K.; Luna, T.; Richardson, B.A.; Kilkenny, F.F.; Runyon, J.B. 2015. Conserving and restoring habitat for greater sage-grouse and other sagebrush-obligate wildlife: The crucial link of forbs and sagebrush diversity. Native Plants Journal. 16(3): 276-299.
Eldredge, E.; Novak-Echenique, P.; Heater, T.; Mulder, A.; Jasmine, J. 2013. Plants for pollinator habitat in Nevada. Tech. Note NV 57. Reno, NV: U.S. Department of Agriculture, Natural Resources Conservation Service. 65 p.
European Native Seed Conservation Network [ENSCONET]. 2009. ENSCONET seed collecting manual for wild species. Edition 1: 32 p.
Farr, D.F.; Rossman, A.Y. 2017. Fungal databases, U.S. National Fungus Collections. U.S. Department of Agriculture, Agricultural Research Service. https://nt.ars-grin.gov/fungaldatabases/.
Franklin, J.F.; Dyrness, C.T. 1973. Natural vegetation of Oregon and Washington. Gen. Tech. Rep. PNW-8. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 452 p.
Hitchcock, C.L.; Cronquist, A. 2018. Flora of the Pacific Northwest: An illustrated manual. Second Ed. Giblin, D.E.; Legler, B.S.; Zika, P.F.; Olmstead, R.G., eds. Seattle, WA: University of Washington Press. 882 p.
Howe, G.; St, Clair, B.; Bachelet, D. 2017. Seedlot Selection Tool. Corvallis, OR: Conservation Biology Institute. https://seedlotselectiontool.org/sst/
Huntly, N.; Bangert, R.; Hanser, S.E. 2011. Native and exotic plants of fragments of sagebrush steppe produced by geomorphic processes versus land use. Plant Ecology. 212(9): 1549-1561.
James, D.G.; Seymour, L. 2018. Development and survival of Chlosyne acastus sterope (Lepidoptera: Nymphalidae) larvae on three host plants in south central Washington. The Journal of the Lepidopterists’ Society. 72(3): 181-184.
Johnson, C.G.; Swanson, D.K. 2005. Bunchgrass plant communities of the Blue and Ochoco Mountains: A guide for managers. Gen. Tech. Rep. PNW-GTR-641. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 119 p.
Lloyd, D.; Angove, K.; Hope, G.; Thompson, C. 1990. A guide to site identification and interpretation for the Kamloops Forest Region. Victoria, BC: British Columbia Ministry of Forests, Research Branch. 399 p.
Loik, M.E.; Edar, S.P. 2003. Microclimate, freezing tolerance, and cold acclimation along an elevation gradient for seedlings of the Great Basin desert shrub, Artemisia tridentata. Journal of Arid Environments. 54(4): 769-782.
Luna, T.; Mousseaux, M.R.; Dumroese, R.K. 2018. Common native forbs of the northern Great Basin important for greater sage-grouse. Gen. Tech. Rep. RMRS-GTR-387. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station; Portland, OR: U.S. Department of the Interior, Bureau of Land Management, Oregon-Washington Region. 76 p.
McInnis, M.L. 1985. Ecological relationships among feral horses, cattle, and pronghorn in southeastern Oregon. Corvallis, OR: Oregon State University. Thesis. 166 p.
Mee, W.; Barnes, J.; Kjelgren, R.; Sutton, R.; Cerny, T.; Johnson, C. 2003. Water wise: Native plants for Intermountain landscapes. Logan, UT: Utah State University Press. 220 p.
Miliczky, E.; Horton, D.R. 2011. Occurrence of the western flower thrips, Frankliniella occidentalis, and potential predators on host plants in near-orchard habitats of Washington and Oregon (Thysanoptera: Thripidae). Journal of the Entomological Society of British Columbia. 108: 11-28.
Moerman, D. 2003. Native American ethnobotany: A database of foods, drugs, dyes, and fibers of Native American peoples, derived from plants. Dearborn, MI: University of Michigan. http://naeb.brit.org
Munz, P.A.; Keck, D.D. 1973. A California flora and supplement. Berkeley, CA: University of California Press. 1905 p.
Nesom, G.L. 2006. 186. Erigeron. In: Flora of North America Editorial Committee, ed. Flora of North America North of Mexico. Volume 20 Magnoliophyta: Asteridae, part 7: Asteraceae, part 2 Asterales, part 2 (Aster order). New York, NY: Oxford University Press: 256-348.
Nesom, G.L. 2008. Classification of subtribe Conyzinae (Asteraceae: Astereae). Lundellia. 11(8): 8-38.
Nicholson, A.; Hamilton, E.; Harper, W.L.; Wikeem, B.M. 1991. Chapter 8: Bunchgrass zone. In: Meidinger, D.; Pojar, J., eds. Ecosystems of British Columbia. Victoria, B.C.: Ministry of Forests and Range Research Branch. 342 p.
Ogle, D.; Pavek, P.; Fleenor, R.; Stannard, M.; Dring, T.; Cane, J.; Fullen, K.; St. John, L.; Tilley, D. 2011. Plants for pollinators in the Inland Northwest. Tech. Note 2B. Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 65 p.
Oldemeyer, J.L.; Martin, S.J.; Woodis, S.G. 1983. A preliminary report on the effects of a deferred-rotation grazing system on wildlife at the Sheldon National Wildlife Refuge. Cal-Neva Wildlife Transactions 1983: 26-42.
Omernik, J.M. 1987. Ecoregions of the conterminous United States. Map (scale 1:7,500,000). Annals of the Association of American Geographers. 77(1): 118-125.
Pavek, P.; Erhardt, B.; Heekin, T.; Old, R. 2012. Forb seedling identification guide for the Inland Northwest: Native, introduced, invasive, and noxious species. Pullman, WA: U.S. Department of Agriculture, Natural Resources Conservation Service, Pullman Plant Materials Center. 144 p.
Plant Conservation Alliance [PCA]. 2015. National seed strategy for rehabilitation and restoration 2015-2020. Washington, DC: U.S. Department of the Interior, Bureau of Land Management. 52 p.
Rhodes, E.C.; Bates, J.D.; Sharp, R.N.; Davies, K.W. 2010. Fire effects on cover and dietary resources of sage-grouse habitat. The Journal of Wildlife Management. 74(4): 755-764.
Royal Botanic Gardens Kew [RBG Kew]. 2017. Seed Information Database (SID). Version 7.1. http://data.kew.org/sid/
Sapsis, D.B. 1990. Ecological effects of spring and fall prescribed burning on basin big sagebrush/Idaho fescue-bluebunch wheatgrass communities. Corvallis, OR: Oregon State University. Thesis. 106 p.
Scianna, J.D. 2004. Blending dry seeds clean. Native Plants Journal. 5(1): 47-48.
SEINet – Regional Networks of North American Herbaria Steering Committee [SEINet]. 2017. SEINet Regional Networks of North American Herbaria. https://symbiota.org/seinet
Shiflet, T.N. 1994. Rangeland cover types of the United States. Denver, CO: Society for Range Management. 152 p.
Solbrig, O.T.; Anderson, L.C.; Kyhos, D.W.; Raven, P.H. 1969. Chromosome numbers in Compositae VII: Astereae III. American Journal of Botany. 56(3): 348-353.
Strother, J.L. 1972. Chromosome studies in western North American Compositae. American Journal of Botany. 59(3): 242-247.
Taylor, R.J. 1992. Sagebrush country: A wildflower sanctuary. Missoula, MT: Mountain Press Publishing Company. 211 p.
Tueller, P.T. 1962. Plant succession on two Artemisia habitat types in southeastern Oregon. Corvallis, OR: Oregon State University. Thesis. 249 p.
USDA Forest Service, Bend Seed Extractory [USDA FS BSE]. 2017. Nursery Management Information System Version 4.1.11. Local Source Report 34-Source Received. Bend, OR: U.S. Department of Agriculture, Forest Service, Bend Seed Extractory.
USDA Forest Service, Great Basin Native Plant Project [USFS GBNPP]. 2014. Seed weight table calculations made in-house. Report on file. Boise, ID: U.S. Department of Agriculture, Forest Service, Boise Aquatic Sciences Laboratory. Available: https://www.fs.fed.us/rm/boise/research/shrub/Links/Seedweights.pdf
USDA Forest Service, Western Wildland Environmental Threat Assessment Center [USFS WWETAC]. 2017. TRM Seed Zone Applications. Prineville, OR: U.S. Department of Agriculture, Forest Service, Western Wildland Environmental Threat Assessment Center. https://www.fs.fed.us/wwetac/threat-map/TRMSeedZoneMapper.php
USDA Natural Resources Conservation Service [USDA NRCS]. 2017. The PLANTS Database. Greensboro, NC: U.S. Department of Agriculture, Natural Resources Conservation Service, National Plant Data Team. https://plants.usda.gov/java
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2016. Bureau of Land Management technical protocol for the collection, study, and conservation of seeds from native plant species for Seeds of Success. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program. 37 p.
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2017. Seeds of Success collection data. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program.
USDI Environmental Protection Agency [USDI EPA]. 2017. Ecoregions. Washington, DC: U.S. Department of the Interior, Environmental Protection Agency. https://www.epa.gov/eco-research/ecoregions
USDI Geological Survey [USDI USGS]. 2017. Biodiversity Information Serving Our Nation (BISON). U.S. Geological Survey. https://bison.usgs.gov/#home
Utah Crop Improvement Association [UCIA]. 2015. How to be a seed connoisseur. Logan, UT: UCIA, Utah Department of Agriculture and Food, Utah State University and Utah State Seed Laboratory. 16 p.
Welsh, S.L.; Atwood, N.D.; Goodrich, S.; Higgins, L.C., eds. 2015. A Utah Flora. Fifth Edition, revised. Provo, UT: Brigham Young University. 990 p.
Westcott, R.L.; Raschko, M. 2018. New plant associations for adults of some species of Acmaeodera Eschscholtz (Coleoptera: Buprestidae) occurring in the western United States. Insecta Mundi. 0644: 1-3.
Young, J.A.; Young, C.G. 1986. Collecting, processing and germinating seeds of wildland plants. Portland, OR: Timber Press. 236 p.
Young, S.A.; Schrumpf, B.; Amberson, E. 2003. The Association of Official Seed Certifying Agencies (AOSCA) native plant connection. Moline, IL: AOSCA. 9 p. Available: https://seedcert.oregonstate.edu/sites/seedcert.oregonstate.edu/files/pdfs/aoscanativeplantbrochure.pdf
Young, V.; Marks, D. 2009. Okanagan grassland species inventory: Multi-species report. Pentiction, B.C.: Ministry of Environment. 18 p.
How to Cite
Gucker, Corey L.; Shaw, Nancy L. 2019. Desert yellow fleabane (Erigeron linearis). In: Gucker, C.L.; Shaw, N.L., eds. Western forbs: Biology, ecology, and use in restoration. Reno, NV: Great Basin Fire Science Exchange. Online: https://westernforbs.org/species/desert-yellow-fleabane-erigeron-linearis/