Authorship
Gucker, Corey; Shaw, Nancy
Publication Date
September 2019
Nomenclature
Sulphur-flower buckwheat (Eriogonum umbellatum) belongs to the Polygonaceae family, Eriogonoideae subfamily, and Oligofonum subgenus (Reveal 2003, 2005). Nomenclature for subtaxa and synonyms follows Reveal (2005).
Family
Polygonaceae – Buckwheat family
Genus
Eriogonum
Species
umbellatum
NRCS Plant Code
ERUM (USDA NRCS 2017).
Subtaxa
The sulphur-flower buckwheat species “consists of a bewildering assemblage of morphologically differing subgroups, some of which have geographical or ecological correlation” (Welsh et al. 2016). Reveal (2005) describes 41 varieties in The Flora of North America (see Appendix 1 for brief descriptions of all varieties; see Appendix 2 for the varieties and their synonyms). Later, however, Reveal (2014, unpublished) described only 21 varieties, suggesting accurate descriptions of the variability in the E. umbellatum complex may require genetic analyses. For this review, nomenclature follows the published work of Reveal (2005).
Common Names
Sulphur-flower buckwheat, sulphur flower, umbrella plant, umbrella desert buckwheat (Craighead et al. 1963; Taylor 1992; Reveal 2005).
Chromosome Number
Chromosome number is: 2n = 80 (Reveal 2005; Welsh et al. 2016).
Hybridization
Most, if not all, sulphur-flower buckwheat varieties hybridize. Where varieties overlap, hybrid swarms and intermediate forms are possible (Hickman 1993; Johnson et al. 2017).
Distribution
Sulphur-flower buckwheat is a widely distributed western species occurring from British Columbia and Alberta south through south-central Colorado, the northern half of Arizona, and California. Appendix 1 provides the distributions of each of the sulphur-flower buckwheat varieties.
Habitat And Plant Associations
Sulphur-flower buckwheat habitats include grasslands (Fig. 1), shrublands, woodlands (Fig. 2), and forests (Fig. 3) from near sea level to above treeline (Rose et al. 1998). It occurs frequently on dry, sandy to rocky sites with low to moderate annual precipitation (≥ 10 in [250 mm]). Plants are often widely scattered but can be very abundant in some stands (USDA FS 1937; Rose et al. 1998; Ogle et al. 2012).
Several studies illustrate sulphur-flower buckwheat’s broad distribution, habitats, and plant associations. At Crater Lake National Park, in southern Oregon, sulphur-flower buckwheat occurred in all life zones (Transition, Canadian, and Hudsonian). It occurred in deserts between 5,500 and 6,750 ft (1,700–2,100 m) with extreme aridity and frigidity. It occurred in low-elevation ponderosa pine (Pinus ponderosa) forests, on pumice slopes of nonforested lower elevation communities (6,250–7,500 ft [1,900-2,300 m]), and on exposed sites in whitebark pine (P. albicaulis) forests above 7,500 ft (2,300 m) (Wynd 1941). At Craters of the Moon National Monument in south-central Idaho, sulphur-flower buckwheat occurred on young lava flows with very little vegetation cover, in later seral shrubland vegetation dominated by mountain big sagebrush (Artemisia tridentata subsp. vaseyana) and antelope bitterbrush (Purshia tridentata), and in the late-seral limber pine (Pinus flexilis) forests with the greatest total vegetative cover (Day and Wright 1985). In the Glass Mountain region of south-central Mono County, California, sulphur-flower buckwheat was common and widespread from 7,200 to 10,500 ft (2,200–3,200 m). It was best developed on rocky granite substrates in mid-elevation mountain big sagebrush (A. tridentata subsp. vaseyana) habitats and dominant in singleleaf pinyon (P. monophylla) woodlands on low rocky slopes and ridges (Horner 2001).
Grasslands. Sulphur-flower buckwheat is a common feature of grassland communities across the West (Fig. 1). A bluebunch wheatgrass (Pseudoroegneria spicata)-sulphur flower buckwheat plant community occurred on Columbia River basalts above 5,000 ft (1,500 m) on moderate to steep slopes in the northern Blue Mountains of northeastern Oregon (Johnson and Swanson 2005). These communities, characteristic of hot, dry upland conditions, had 45 to 75% rock and gravel cover (Johnson and Swanson 2005; Powell et al. 2007). In 10 years of surveying southwestern and northeastern exposure grasslands in southwestern Montana’s Gravelly Range, sulphur-flower buckwheat only occurred on northeastern exposures dominated by western needlegrass (Achnatherum occidentale subsp. occidentale). Northeastern slopes were wetter than the southwestern slopes dominated by Idaho fescue (Festuca idahoensis) and bluebunch wheatgrass (Mueggler 1983). At elevations of 7,000 to 9,000 ft (2,100–2,700 m) in the Madison Range, also in southwestern Montana, sulphur-flower buckwheat occurred most often in openings when forested, non-forested, and ecotone vegetation were compared. Idaho fescue was characteristic of non-forested and ecotone sites (Patten 1963). When dry grasslands along an altitudinal gradient from 5,300-11,000 ft (1,600–3,400 m) were evaluated in Colorado’s Boulder and Gilpin counties, sulphur-flower buckwheat occurred in all but the highest subalpine-elevation grasslands (Ramaley 1916).

Figure 1. Sulphur-flower buckwheat growing in a forest grassland opening in Arizona. Photo: USDI BLM AZ 932 SOS.
Shrublands/woodlands. In descriptions of rangeland cover types of the western US, sulphur-flower buckwheat was frequent in antelope bitterbrush-Idaho fescue, mountain big sagebrush, and western juniper (Juniperus occidentalis)/big sagebrush/bluebunch wheatgrass communities (Shiflet 1994). In descriptions of natural vegetation in Oregon and Washington, sulphur-flower buckwheat was characteristic of rock garden communities associated with rocky outcrops, western juniper/big sagebrush/threadleaf sedge (Carex filifolia), and antelope bitterbrush/western wheatgrass communities. In Grand Teton National Park, Wyoming, sulphur-flower buckwheat occurred in all 31 evaluated shrubland stands dominated by low sagebrush (A. arbuscula), mountain big sagebrush, and/or antelope bitterbrush (Sabinske and Knight 1978). In south-central Wyoming, sulphur-flower buckwheat was common in quaking aspen (Populus tremuloides) stands growing on leeward high-snow accumulation slopes or downslope from large snow drifts. These stands received heavy deer (Odocoileus spp.) and cattle use (Burke et al. 1989).

Figure 2. Sulphur-flower buckwheat growing in a juniper woodland in Utah. Photo: US Forest Service, Provo Shrub Laboratory.
Sulphur-flower buckwheat is dominant or characteristic in several Great Basin woodlands including Utah juniper/Gambel oak (J. osteosperma/Quercus gambelii) in western Utah and eastern Nevada, singleleaf pinyon/Wyoming big sagebrush (A. tridentata subsp. wyomingensis) in southwestern Utah and southeastern Nevada, and singleleaf pinyon/mountain big sagebrush and singleleaf pinyon/curl-leaf mountain mahogany (Cercocarpus ledifolius) in the extreme northern portion of the Mojave Desert (West et al. 1998). In an extensive survey of vegetation at the Nevada Test Site and south-central Nevada, four sulphur-flower buckwheat varieties (dichrocephalum, subaridum, vernum, and versicolor) were locally common in black sagebrush (A. nova), big sagebrush, big sagebrush-mountain mahogany, and big sagebrush-singleleaf pinyon-Utah juniper vegetation at elevations of 4,500 to 9,000 ft (1,400–2,700 m) (Beatley 1976). In a successional study of singleleaf pinyon-Utah juniper woodlands comparing 21 sites burned 1 to 60 years prior, sulphur-flower buckwheat occurred on 52% of burned stands. It was more common on east and north than south and west slopes and more frequent on sites that were not seeded than those that were following fire (Koniak 1985). In Underdown Canyon in Nevada’s Shoshone Mountains, sulphur-flower buckwheat was an indicator species for the big sagebrush-Idaho fescue understory community type in single-leaf pinyon woodland habitats. As tree cover increased, sulphur-flower buckwheat disappeared from the understory (Urza et al. 2017).
Forests/alpine. Sulphur-flower buckwheat occurs in conifer forests throughout its range. It is common in lodgepole pine/western wheatgrass (Pinus contorta/Pascopyrum smithii) vegetation in central Oregon (Franklin and Dyrness 1973), ponderosa pine-white fir (Abies concolor) forests in Nevada (Beatley 1976), low-elevation, xeric western white pine (P. monticola) forests in the Siskiyou Mountains of Oregon and California (Whittaker 1960), and ponderosa pine and mixed ponderosa pine-Douglas-fir (Pseudotsuga menziesii) forests on the east side of the Colorado Front Range (Kooiman and Linhart 1986).
Sulphur-flower buckwheat is considered a dominant species in subalpine and alpine habitats. The Payson’s sedge (Carex paysonis)-sulphur-flower buckwheat community occurs above treeline on the south side of Mt Hood in Oregon (Titus and Tsuyuzaki 1999). On the east slope of the Sierra Nevada in California, sulphur-flower buckwheat dominates treeline habitats on south and southwest slopes from 7,840 to 9,120 ft (2,390–2,780 m) (Jackson 1985).

Figure 3. Sulphur-flower buckwheat growing in a subalpine environment in Nevada. Photo: USDI BLM NV 030 SOS.
Elevation
Sulphur-flower buckwheat occupies sites ranging from 300 to 12,100 ft (100–3,700 m); see Appendix 1 for the elevation range of each of the sulphur-flower buckwheat varieties. Combining elevation and distribution information can be useful in distinguishing varieties in some regions.
Soils
In sulphur-flower buckwheat habitats, soils are often coarse-textured and dry, but parent material, nutrient content, and pH can vary widely. Cover of sulphur-flower buckwheat was greatest (6.8%) and its presence useful in discriminating the next to driest sagebrush meadow along a moisture gradient in the northwest corner of the Greater Yellowstone Ecosystem. The moisture gradient ranged from sedge marshes with some standing water to south-facing rocky sagebrush (Debinski and Kindscher 1994). In central Colorado grasslands where sulphur-flower buckwheat occurred, soils were generally coarse-grained, quick-drying, and averaged 7% water content (Ramaley 1916). In Rocky Mountain National Park, Colorado, sulphur-flower buckwheat was common in dry, sandy, or rocky soils on south-facing foothill and montane regions (Holch et al. 1941). In Grand Teton National Park, Wyoming, sulphur-flower buckwheat occurred in sagebrush shrublands on soils with 25 to 50% clay, 33 to 40% silt, 31 to 37% sand, and 3.7 to 6.4% organic matter (Sabinske and Knight 1978).
Sulphur-flower buckwheat tolerates shallow soils. At Craters of the Moon National Monument in south-central Idaho, sulphur-flower buckwheat occurred in mountain big sagebrush-needle and thread (Hesperostipa comata) communities occupying sandy shallow soils (Day and Wright 1985). In northern Colorado, sulphur-flower buckwheat occurred in just 1 of 10 needle and thread-blue grama-sideoats grama (Bouteloua gracilis–B. curtipendula) stands evaluated. It occurred in the stand with the shallowest soils (8 in [20 cm]) and highest sand content (72%) (Hanson and Dahl 1957).
Sulphur-flower buckwheat occurs on soils derived from various parent materials. When vegetation-soil relationships were evaluated in the White Mountains of eastern California, sulphur-flower buckwheat occurred on all substrates: dolomite, basalt, sandstone, and adamellite. It was most common on sandstone with the greatest average soil moisture (moisture gradient: sandstone > dolomite > basalt > adamellite) (Marchand 1973). In shortgrass vegetation at the Central Plains Experiment Range in Weld County, Colorado, sulphur-flower buckwheat was characteristic of shale/sandstone breaks and ravines (Hazlett and Sawyer 1998).
Although found on a variety of soil types, sulphur-flower buckwheat does show soil preferences in some regions. In the Siskiyou Mountains in Oregon and California, sulphur-flower buckwheat occurred on serpentine, diorite, quartzite, and argillite soils (Whittaker 1960). It is also found on serpentine soils in California. These soils have critically low levels of nitrogen, phosphorus, potassium, and calcium; high levels of magnesium and iron; and traces of toxic elements like chromium, nickel, and cobalt (Safford et al. 2005). In the Bear River Range of northeastern Utah and southeastern Idaho, sulphur-flower buckwheat was characteristic of and almost entirely restricted to dolomite soils when dolomite and quartzite soils were compared. Soil pH, silt content, and percent moisture were significantly greater (P < 0.01) and sand content was significantly lower for dolomite than quartzite soils. Dolomite soils averaged 44% sand, 42% silt, and 14% loam with an average pH of 7 (range: 6.2–8.3) (Neely and Barkworth 1984). In the dry, cold, high-elevation bristlecone pine (Pinus aristada) zone in the White Mountains of California, sulphur-flower buckwheat occurred in stands on sandstone and granite but not on dolomite soils (Table1; Wright and Mooney 1965).
Table 1. Characteristics of soils in the dry, cold, bristlecone pine zone (9,500-11,500 ft [2,900–3,500 m]) in the White Mountains of California. Sulphur-flower buckwheat occurred only in stands on sandstone and granite soils (Wright and Mooney 1965).
|
Parent material |
Sand |
Silt |
Clay |
Available moisture |
pH |
|
—————–%—————- |
|||||
|
Sandstone |
63 |
33 |
4 |
25 |
6.2–6.4 |
|
Granite |
82 |
15 |
3 |
16 |
5.9–6.2 |
|
Dolomite |
64 |
34 |
2 |
20 |
8.0–8.1 |
Description
Sulphur-flower buckwheat is an extremely variable species, with different varieties growing as herbaceous (Fig. 4) or woody perennials from a woody branching crown (Dayton 1960; Rose et al. 1998; Lambert 2005; Reveal 2005). Plants range from small, low-growing and spreading to rather large, erect shrub forms (1–80 in [2–200 cm] tall × 2-80 in [5-200 cm] wide) (Hickman 1993; Reveal 2005; Hitchcock and Cronquist 2018). Foliage can be glabrous to densely covered in short, woolly hairs (Reveal 2005).

Figure 4. Sulphur-flower buckwheat plant growing in Oregon. Photo: USDI BLM OR014 SOS.
Belowground description. The sulphur-flower buckwheat root system ranges from a semi-taproot (Woolley 1936) to a strong, woody taproot (Rose et al. 1998; Lambert 2005), reaching only shallow depths (Ramaley 1916) or up to 39 in (100 cm) deep with considerable biomass (Woolley 1936; Rau et al. 2008). Two sulphur-flower buckwheat plants excavated from the Boise River Watershed in Idaho had semi-taproot systems with slender main vertical roots and many slender horizontal branches. Roots penetrated to a maximum depth of 28 in (70 cm) and 39 in (100 cm). Spread of the longest horizontal roots was about 16 in (40 cm) and 20 in (50 cm) (Woolley 1936). A single plant excavated from Rocky Mountain National Park, Colorado, had a main root that tapered to its minimum diameter at about 8 in (20 cm) deep, but this small diameter root extended to 41 in (104 cm) deep. The root was tough and dark brown with heavy bark allowing it to penetrate the hard, rocky soil. The numerous lateral roots extended a maximum of 48 in (122 cm) (Holch et al. 1941).
Aboveground description. Flowering stems range from spreading to erect. Stems are slender with leaves mostly basal, except for a few bracts or a whorl of bracts below the umbel (Reveal 2005; Pavek et al. 2012). Non-flowering branches and leaves are persistent (Lambert 2005). Leaves are loose to compact in basal rosettes (Welsh et al. 2016). Leaf blades are oval to elliptic, 0.5 to 1.5 in (1.3–3.8 cm) long and about half to a third as wide with short petioles (0.4–1.6 in [1–4 cm] long), (Pavek et al. 2012; Welsh et al. 2016; Hitchcock and Cronquist 2018). Leaves can appear bright green and hairless to white woolly with dense hairs, especially on the undersides (Fig. 5) (Hickman 1993; Reveal 2005; Welsh et al. 2016).
Inflorescences are compact or compound umbels (Fig. 6) having long stalks terminating in ball-like clusters of 2 to 10 tiny flowers. Individual flowers have six petal-like segments (tepals) ranging from bright yellow to cream or reddish in color (Hickman 1993; Shaw 1995; Reveal 2005; Pavek et al. 2012). Individual flowers are held in five-toothed cups (involucres) (Shaw 1995). Flowering stems and floral tissue often persist through summer (Monsen et al. 2004). Plants often produce perfect flowers together with either male or female flowers (Reveal 2005). Sulphur-flower produces single-seeded achenes that are brown, three-sided, 2 to 7 mm long, and glabrous except for a sparsely hairy beak (Reveal 2005; Shaw 1995; Welsh et al. 2016).


Figure 5. Sulphur-flower buckwheat leaves are fuzzy to glabrous on both or just one side. Photos: USDI BLM NV040 SOS (upper), USDI BLM CPI SOS (lower).
Some of the variability in sulphur-flower buckwheat forms is partitioned among the multiple varieties (see Appendix 1). Other variation can result from changes in weather or climate. In an experimental study in Grand Teton National Park, the height of sulphur-flower buckwheat inflorescences was 3 in (7.8 cm) greater with snow removal treatments and 3.7 in (9.5 cm) greater with snow removal + heating treatments in 1 of 2 years. Snow removal treatments significantly reduced soil moisture (P ≤ 0.02). Heating increased morning temperatures and increased soil moisture early in the growing season (Sherwood et al. 2017).
Reproduction
Sulphur-flower buckwheat reproduces from seed. Plants often produce perfect flowers together with either male or female flowers (Reveal 2005).

Figure 6. Sulphur-flower buckwheat inflorescences contain many small individual flowers having cream to yellow to red petals. Photo: USDI BLM WY050 SOS.
Pollination
Pollinator visitation increases seed production. On Arrastre Flat in the San Bernardino Mountains of southern California, plants protected from insects produced just 25% of the seed produced by freely visited plants. Regular visitors of sulphur-flower buckwheat flowers were Sphecid wasps (Sphecidae) and honey bees (Apis mellifera). Occasional visitors included flies, other bees, and butterflies (O’Brien 1980). Sulphur-flower buckwheat is important to and pollinated by a variety of flies, bees, wasps, and butterflies, see Insects section in Wildlife and Livestock Use.
Ecology
Sulphur-flower buckwheat is an early colonizer of disturbed sites (Majerus 1991; Monsen et al. 2004). It is common in early to mid-seral communities (Bunting et al. 1999) and often restricted to openings in late-seral communities with high tree cover (Figs. 2 and 3) (Urza et al. 2017). When top-killed by fire or other disturbances, it often takes 2 or more years to recover to prefire abundance levels (Fraas 1992; Rau et al. 2008).
Sulphur-flower buckwheat is an early colonizer of disturbed and harsh sites, and its establishment may facilitate recruitment of later seral species (Majerus 1991; Meyer 2008). At Craters of the Moon National Monument in south-central Idaho, seed densities and soil nitrogen were higher beneath sulphur-flower buckwheat canopies than in vegetation interspaces. The prostrate growth form may have trapped wind-blown seed and litter, which increased soil nitrogen. Other plants were not positively associated with sulphur-flower buckwheat but were with cushion buckwheat (E. ovalifolium), the initial colonizer that also has a prostrate growth form (Day and Wright 1989). On the east slope of the Sierra Nevada Mountains in California, Clark’s nutcrackers (Nucifraga columbiana) cached whitebark pine seeds at the base of sulphur-flower buckwheat plants (Tomback 1982). More whitebark pine seedlings were found near sulphur-flower buckwheat plants than expected by chance. Tomback (1982) suggested sulphur-flower buckwheat provided cooler, moister conditions during the early heat-sensitive stage of seedling growth.
In woodland or forest ecosystems, sulphur-flower buckwheat is most common where tree cover is absent or low. In a mountain big sagebrush-western juniper mosaic in the Owyhee Mountains of Idaho, sulphur-flower buckwheat was primarily associated with early to mid-seral conditions when 40 plots (0.62 ac [0.25 ha]) ranging from recently burned grasslands to western juniper woodlands (>500 yrs) were compared (Bunting et al. 1999). In Underdown Canyon in Nevada, sulphur-flower buckwheat was absent from communities with high tree cover (singleleaf pinyon or Utah juniper) (Urza et al. 2017). When thinned and burned dry forests in northeastern Oregon were evaluated, sulphur-flower buckwheat was associated with dry, sunny sites (Youngblood et al. 2006). Production of sulphur-flower buckwheat was greater on chained than untreated sites in mountain big sagebrush-Utah juniper vegetation in central Utah. Sulphur-flower buckwheat production was 6.3 lbs/ac (7.1 kg/ha) on treated and 2.3 lbs/ac (2.6 kg/ha) on untreated plots 5 years after sites were chained and seeded with nonnative grasses and alfalfa (Medicago sativa) (Clary 1989).
Seed And Seedling Ecology
Seed production and germination potential vary by year. When wildland seed was collected from western Nevada and eastern California over a period of 15 years, seed production was low to none in 6 of the years. Maximum germination in some years reached 90% but in other years was just 50% (Young 1989). Monsen et al. (2004) reported that sulphur-flower buckwheat seedlings are persistent and competitive; however, young seedlings are small, especially when water is limited (Peterson and Billings 1982; Parkinson and Zabinksi 2009).
In a greenhouse study, the relative growth rate (RGR) of sulphur-flower buckwheat seedlings was 0.38 mg/mg/week for shoots, 0.48 mg/mg/week for roots, and 0.40 mg/mg/week overall. All seedling roots were extremely fine (<1 mm diameter) with just 2 or 3 branches within 1 to 2 in (3-5 cm) of the soil surface. Seedling biomass averaged 0.04 g at 6 weeks, 0.16 g at 9 weeks, and 0.46 g at 12 weeks. Seedling biomass at 12 weeks was at least 3 times less than other native species evaluated (hoary tansyaster [Dieteria canescens], royal penstemon [Penstemon speciosus], and Munro’s globemallow [Sphaeralcea munroana]) but much greater than the biomass of bigseed biscuitroot (Lomatium macrocarpum) seedlings. Biomass of sulphur-flower buckwheat seedlings was relatively unchanged when grown with Sandberg’s bluegrass (Poa sandbergii), or squirreltail (Elymus elymoides). Seedling biomass was reduced when grown with cheatgrass (Bromus tectorum) (P<0.001) (Parkinson 2008; Parkinson et al. 2013).
In other studies, availability of water appeared to improve seedling growth. In field trials, sulphur-flower buckwheat seedlings emerged in late March when seeding occurred the previous fall at two sites (Lucky Peak and Orchard) near Boise, Idaho. By late July, the average biomass of sulphur buckwheat seedling shoots was 3.9 g at Lucky Peak and 0.25 g at Orchard. Annual precipitation averages 14 in (361 mm) at Lucky Peak and 11.5 in (292 mm) at Orchard (Parkinson and Zabinksi 2009). In a controlled experiment, sulphur-flower buckwheat seed collected from dry alpine ridges of the Sierra Nevada was grown in a sandy medium and watered either daily or weekly. The dry weight of sulphur-flower buckwheat plants was significantly lower in weekly (1 g) than in daily (1.6 g) water treatments at 111 days after seeding (P<0.05). The RGR was also significantly lower in weekly (0.04 g/g/day) than in daily (0.05 g/g/day) treatments (P<0.05), but root:shoot biomass ratios were not different between weekly and daily watering treatments (Peterson and Billings 1982).
Disturbance Ecology
Existing sulphur-flower buckwheat plants are often damaged by disturbance, and the recovery period is often longer with increased disturbance frequency or intensity. Sulphur-flower buckwheat was rated as having moderate resistance, low short-term resilience, and moderate long-term resilience following a field experiment in the Bob Marshall Wilderness in Montana. Lanes within existing vegetation were trampled by people (130-190 lbs [60-90 kg]) wearing lug-soled boots 5 to 1,600 times/year for up to 3 years. Frequency of sulphur-flower buckwheat was reduced to 50% of pre-trampling levels after 300 to 400 passes. Less than 10% of sulphur-flower buckwheat’s relative cover was recovered between the end of the first and the start of next trampling (10 mos). Relative cover increased by 10 to 30% by 3 years after the last season of trampling (Cole 1988).
Sulphur-flower buckwheat growing points are located just above the soil surface, making them susceptible to damage by fire (Miller et al. 2013). At least short-term reductions in the abundance of sulphur-flower buckwheat are possible following fire.
Sulphur-flower buckwheat sprouts may not be seen in the first year after fire and recovery can take 10 years or more. Sulphur-flower buckwheat sprouted in the second but not the first post-fire year after a spring prescribed fire in mountain big sagebrush/singleleaf pinyon vegetation in Nevada’s Shoshone Mountains (Rau et al. 2008). The fire produced surface temperatures of 403 to 696 °F (206–369 °C), 0.8-in (2 cm) deep temperatures of 104 to 187 °F (40–86 °C), and 2-in (5 cm) deep temperatures of 104 to 111 °F (40–44 °C) (Rau et al. 2007). Sulphur-flower buckwheat dominated a subalpine site 5 years following a fire with moderate burn conditions in the southern Sierra Nevada (Anjoziam 2008). In bitterbrush shrublands in southwestern Montana, frequency of sulphur-flower buckwheat was nearly equal on 10-year-old burned and unburned plots. The site was rested from livestock use for 1 year before burning to increase fuel loads. Burning occurred on November 3 when relative humidity was 37%, air temperature was 52 °F (11 °C), and wind speeds were 56 to 95 ft (17–29 m)/second (Fraas 1992).
Many sulphur-flower buckwheat plants failed to sprout in the first post-fire year following experimental burning of seed production plots in Ontario, Oregon. Senescing plants (late August 2012) were burned at various heat intensities using a propane torch and burn barrel. About 60% of plants sprouted in the first year when burning produced low (466 °F [241 °C]) or very low (212 °F [100 °C]) soil surface temperatures. Less than 20% sprouted in the first post-fire year when burning produced soil surface temperatures of 572 °F (300 °C), 932 °F (500 °C), or 1,112 °F (600 °C). Of the 188 plants evaluated, all 27 unburned plants survived and just 48 (30%) burned plants sprouted in the first post-fire year and 22 of those failed to flower. First-year post-fire sprouts that flowered produced significantly fewer umbels than unburned plants (P < 0.0001) (Love and Cane 2019).
Sulphur-flower buckwheat cover did not reach unburned levels by the third year following a spring or the second year following a late summer prescribed fire in mountain big sagebrush in Wyoming’s Bridger-Teton National Forest. The spring fire burned patchily on June 3. The late summer fire burned with complete consumption on August 27 (Table 2; McGee 1976).
Table 2. Cover and frequency of sulphur-flower buckwheat on burned and unburned plots in mountain big sagebrush in Wyoming’s Bridger-Teton National Forest (McGee 1976).
|
Postfire year |
Spring burn |
Summer burn |
Unburned |
|
1* |
0.1 (3) |
3.6 (20) |
3.0 (20) |
|
2 |
0.2 (6) |
— |
3.1 (15) |
|
3 |
1.3 (14) |
0.5 (4) |
4.2 (23) |
*Prefire value for summer burned plots.
Wildlife And Livestock Use
Buckwheat species provide forage for a variety of ungulates, small mammals, and livestock. Buckwheat seeds and its many insect visitors are important to small mammals, game birds, and song birds (Martin et al. 1951; Monsen et al. 2004).
Big game and livestock. Sulphur-flower buckwheat is used by deer and elk (Cervus canadensis) and considered moderately palatable in Idaho (Holmgren 1954). Use by big game and livestock occurs in summer, fall, and winter (Stevens 1966; Johnson et al. 1978; Monsen et al. 2004). Although consumed by a variety of big game and livestock, sulphur-flower buckwheat rarely makes up a large proportion of total diets.
Tame elk frequently fed on sulphur-flower buckwheat on xeric winter range in Rocky Mountain National Park (Hobbs et al. 1981). In the Crow Creek Drainage in Montana’s Elkhorn Mountains, sulphur-flower buckwheat made up 1% of elk summer use, and various buckwheat species made up to 4% of domestic sheep feeding in summer (Stevens 1966). In Yellowstone National Park, utilization of sulphur-flower buckwheat was 5% by bighorn sheep (Ovis canadensis) in early July (Davis cited in Mills 1937). Frequency of sulphur-flower buckwheat was 58% in the microhistological analysis of mountain goat (Oreamnos americanus) rumens collected in the summer in Colorado’s Sawatch Range (Johnson et al. 1978). Sulphur-flower buckwheat was eaten by mountain goats in Montana’s Bitterroot Mountains, but was not the most preferred forb (Smith 1976). It was also utilized at a low level by pronghorn (Antilocapra americana) in Utah (Smith and Beale 1980).
In a review of Rocky Mountain mule deer (Odocoileus hemionus subsp. hemionus) food habits, Kufeld et al. (1973) found that buckwheat species were some of the most frequently consumed forbs reported in all studies evaluated. Forbs, in general, were rarely a large proportion of diets and rarely did a particular forb consistently constitute a major diet component (Kufeld et al. 1973). Sulphur-flower buckwheat made up to 2% of the late summer diets of tame mule deer gazing in the Sheeprock Mountains of western Utah (Austin and Urness 1986) and up to 2% in April and 3% in October diets of tame mule deer grazing in winter range in Piceance Basin in northwestern Colorado (Bartmann 1983). Sulphur-flower buckwheat was one of the forbs eaten most by mule deer in summer 1982 at the Sheldon National Wildlife Refuge (Oldemeyer et al. 1983). Inflorescences were preferred, but leaves were eaten once flowers were dry. More bites were taken from sulphur-flower buckwheat in early summer than in mid- or late summer, and more from within a livestock exclosure than the cattle-grazed area. Production of sulphur-flower buckwheat was greater in the exclosure than the grazed area (Oldemeyer et al. 1983).
Both domestic sheep and cattle utilize sulphur-flower buckwheat. Domestic sheep consume sulphur-flower buckwheat flowers (Dayton 1960). It was preferred by domestic sheep in southwestern Montana and southeastern Idaho (Craighead et al. 1963). On a big game winter range and cattle grazing allotment on the Deerlodge National Forest in southwestern Montana, sulphur-flower buckwheat occurred in deer and cattle exclosures but not on sites receiving use by both deer and cattle (Fraas 1992). In a grazing trial, buckwheat species (sulphur-flower buckwheat and parsnipflower buckwheat [Eriogonum heracleoides]) made up 5% of cattle diets from June 26 to July 16 in the upper Ruby River Valley south of Sheridan, Montana (Ralphs and Pfister 1992).
Small mammals. Although a variety of small mammals feed on sulphur-flower buckwheat seed and forage (Martin et al. 1951; Monsen et al. 2004), species-specific use was rarely reported in the literature. Buckwheat seeds were gathered and consumed by chipmunks (Neotamias minimus) and white-footed mice (Peromyscus leucopus) in southwestern Montana and southeastern Idaho (Craighead et al. 1963). In Sierra Nevada’s Lundy Canyon, pika (Ochotona daurica) hay piles or winter food caches frequently contained sulphur-flower buckwheat flower heads. Lundy Canyon was considered marginal pika habitat (Millar et al. 2013).
Birds. Sulphur-flower buckwheat attracts a variety of insects, making it important to insect-feeding song and game birds (Ogle et al. 2012). On Cold Spring Mountain, in Moffat County, Colorado, sulphur-flower buckwheat was one of the most common forbs encountered along greater sage-grouse (Centrocercus urophasianus)-use transects. This was during a study comparing random greater sage-grouse use, herbicide-treated, and non-treated sites in semi-arid sagebrush (Dunn and Braun 1986). Significantly greater cover of sulphur-flower buckwheat was found in greater sage-grouse use (nesting, brood, and adult habitats) than in non-use areas (P<0.05). The study evaluated sagebrush/grassland and smooth brome (Bromus inermis)-dominated vegetation in the spring and summer in Strawberry Valley, Utah (Bunnell et al. 2004). Areas with at least 5% sulphur-flower buckwheat cover were positively associated with sharp-tailed grouse (Tympanuchus phasianellus) brood sites in various communities near Savery, Wyoming. Frequency of sulphur-flower buckwheat was 88% at sharp-tailed grouse brood sites, 41% at random sites, and 15% at greater sage-grouse brood sites (Klott and Lindzey 1990).
Insects. Sulphur-flower buckwheat is an important nectar source and larval host for a variety of pollinators (James and Nunnallee 2011). It provides bees with late-season nectar (Fig. 7) and attracts predatory wasps (Ammoplanus spp.) (Smith 2008; Ogle et al. 2012). Other insects, including those beneficial to crops, are also attracted to sulphur-flower buckwheat (James et al. 2014b).
Pollinators representing the Andrenidae, Apidae, Bombyliidae, Halictidae, Hesperiidae, Lycaenidae, Muscidae, Nymphalidae, Pieridae, Sphecidae, Syrphidae, and Tachinidae families were observed utilizing sulphur-flower buckwheat in at least 1 of 3 years of observations made in meadows at the Rocky Mountain Biological Laboratory, Colorado (Burkle and Irwin 2009).

Figure 7. Honey bee visits sulphur-flower buckwheat flowers at Oregon State University’s Malheur Experiment Station, Ontario, Oregon. Photo: C. Shock, June 19.
Many species of butterflies use sulphur-flower buckwheat as a nectar source and some utilize it as a larval host. Behr’s hairstreak butterflies (Satyrium behrii) fed on the nectar from various buckwheat species, especially when buckwheat grew near antelope bitterbrush (James and Nunnallee 2011). Sulphur-flower buckwheat was the primary nectar source for a high-altitude butterfly, Clodius parnassian (Parnassius clodius), in dry sagebrush meadows in Grand Teton National Park. In 1 of 2 study years, the number of Clodius parnassians and density of sulphur-flower buckwheat inflorescences were positively related (Auckland et al. 2004). Sulphur-flower buckwheat is also an important nectar source for Leona’s little blue (Philotiella leona), a threatened and severely restricted butterfly occurring in a 12 mi² (32 km²) area of south-central Oregon’s Antelope Desert. Of 276 nectaring records, 33% were on sulphur-flower buckwheat (James et al. 2014a). The cythera metalmark butterfly (Apodemia mormo cythera) is often found associated with sulphur-flower buckwheat (Pratt and Ballmer 1991), but more commonly associated butterflies are the Rocky Mountain dotted-blue (Euphilotes ancilla) and lupine blue (Plebeius lupini) (Reveal 2005).
Sulphur-flower buckwheat is a confirmed larval host for the following butterfly species: Sheridan’s green hairstreak (Callophrys sheridanii), Sheridan’s hairstreak (C. s. neoperplexa), and green hairstreak (C. s. sheridanii), western green hairstreak (C. affinis), American dotted blues (Euphilotes spp.), Glaucon blue (E. glaucon), lupine blue (Plebejus lupini), and Lutz’s blue (P. acmon lutzi) (Ferris 1973; James and Nunnallee 2011).
Other insects attracted to sulphur-flower buckwheat include metallic wood-boring beetles (Agrilus illectus) (Nelson and Westcott 1976), predatory wasps (Ammoplanus alpinensis, A. bifidus, and A. vanyumi) (Smith 2008), and pemphredonine wasps (Pulverro spp.) (Pate 1937). In the Yakima Valley of central Washington, sulphur-flower buckwheat was one of many wild buckwheat species attracting beneficial insects. Sulphur-flower buckwheat was suggested for use in and around crops to lure natural enemies of arthropods for organic or low pesticide management, especially in vineyards (James et al. 2014b).
Nutritional Value
Nectar volume averaged 0.68 µL, and sugar content averaged 344.66 mg for sulphur-flower buckwheat growing in dry sagebrush meadows in Grand Teton National Park. With heating experiments, which increased minimum nighttime temperatures by a few degrees, both nectar volume and sugar content were slightly reduced from control levels (Debinski et al. 2014).
Forage quality information for sulphur-flower buckwheat is provided for a Colorado site (Table 3). Sulphur-flower buckwheat made up a large portion of elk diets and was a high-protein forb.
Table 3. The average structural composition, crude protein content, and in vitro digestibility of sulphur-flower buckwheat plants on a Colorado upper montane range evaluated from November to March from 1976 to 1978 (Hobbs et al. 1981).
|
Year |
Cell wall |
Acid detergent fiber |
Lignin |
Crude protein |
In vitro digestible dry matter |
|
————————–%————————- |
|||||
|
1977 |
43 |
34 |
17 |
7.8 |
30 |
|
1978 |
55 |
43 |
18 |
6.3 |
25 |
Ethnobotany
There are several wide-ranging uses of sulphur-flower buckwheat in the ethnobotany literature (Reveal 2005). The Cheyenne regarded it as a prized medicinal plant for which horses were traded. A strong tea from powdered stems and flowers was drunk to treat prolonged bleeding during menstruation (Hart 1981). Flowers were mashed and mixed with water to make a salve that the Kawaiisu of northern California applied externally to gonorrheal sores (Zigmond 1981). Leaves and sometimes boiled roots were mashed into poultices to treat lameness or rheumatism and a decoction from roots was taken hot for colds and stomach aches by tribes of Nevada (Train et al. 1941). A tea from roots was used to treat colds by the Owens Valley Paiute in northern Nevada (Steward 1933). The Navajo used sulphur-flower buckwheat as an emetic to induce vomiting (Wyman and Harris 1951). Blood and Blackfoot tribes referred to it as “makes your nose bleed” plant and used it to relieve persistent itching and to treat open sores (Ayer et al. 1990). The Klamath Indians of Oregon used the leaves on burns to ease pain and offer protection from the air (Coville 1897).
Horticulture
Sulphur-flower buckwheat is available commercially as a landscape plant (Sutton and Johnson 1974; LBJWC 2019; Plant Select 2019). It is recommended for low maintenance, xeric, cold, and poor soil situations (Monsen et al. 2004; USU Ext. 2017). It has several attributes making it a desirable ornamental plant, including semi-evergreen foliage, good structure, leaves that often turn red in the fall, potential for use as a ground cover, and excellent dry flowers for arrangements. Plants flower for long periods, and flowers remain colorful even when dry (Sutton and Johnson 1974; Parkinson 2003; Dyer 2005; Meyer et al. 2009). Sulphur-flower buckwheat was used as a component of the vegetation in green roofs, which are flat or sloped roofs supporting vegetation to reduce heat island effects, improve storm water management, reduce heat and cooling energy use, and provide wildlife habitat (Dvorak and Volder 2010).
There are several sulphur-flower buckwheat cultivars, ‘Kannah Creek’, ‘Shasta’, and ‘Sierra’ (USU Ext. 2017). ‘Sierra’ was collected from native plants (variety polyanthum) growing in South Lake Tahoe, California (Monsen et al. 2004; Dyer 2005). It was developed for erosion control and landscaping on dry rocky slopes and droughty sites (Young-Matthews 2012). Reveal (2003) reported that many varieties of sulphur-flower buckwheat are or were in cultivation, most of them in Europe: aureum, chlorothamnus, ellipticum, haussknechtii, nevadense, porteri, and umbellatum, versicolor and that many others were worthy of cultivation, mostly for their attractive flowers or forms: ahartii, desereticum, devestivum, dumosum, lautum, glabberrimum, goodmanii, minus, speciosum, stragulum, and vernum.
Revegetation Use
Sulphur-flower buckwheat has several traits making it a useful revegetation species. It tolerates dry and cold growing conditions and has been used successfully in the revegetation of severely disturbed sites (Everett et al. 1980; Monsen et al. 2004). Seedlings are considered persistent and competitive, and plants provide rapid soil stabilization (Monsen et al. 2004). Flowers attract a variety of pollinators and provide important pollinator habitat (Ogle et al. 2007; Eldredge et al. 2013; LBJWC 2019). Buckwheat species are often important pioneers on disturbed sites. Their early establishment may help facilitate recruitment of later seral species, making them useful for revegetation of mined areas, roadways, and more (Meyer 2008). See earlier sections on Ecology and Insects section of Wildlife and Livestock Use.
Developing A Seed Supply
For restoration to be successful, the right seed needs to be planted in the right place at the right time. Coordinated planning and cooperation is required among partners to first select appropriate species and seed sources and then properly collect, grow, certify, clean, store, and distribute seed for restoration (PCA 2015).
Developing a seed supply begins with seed collection from native stands. Collection sites are determined by current or projected revegetation requirements and goals. Production of nursery stock requires less seed than large-scale seeding operations, which may require establishment of agricultural seed production fields. Regardless of the size and complexity of any revegetation effort, seed certification is essential for tracking seed origin from collection through use (UCIA 2015).
Seed Sourcing
The high degree of species variability and results from common garden studies suggest it is important to match the habitats of the plant material source to the revegetation or restoration site (Johnson et al. 2018; Fisk et al. 2019; LBJWC 2019). Consulting a local taxonomist and/or using local material is recommended when developing a revegetation plan or seed mix.
Plant traits were highly variable when 72 populations of sulphur-flower buckwheat were grown in a common garden study at the Western Regional Plant Introduction Station in Pullman, Washington, which suggested strong genetic variation among source populations. Plant traits were most frequently correlated with average annual temperature, differences in the average temperatures of the warmest and coldest months, and 30-year extreme maximum temperatures at source locations. Researchers suggested using multiple sulphur-flower buckwheat populations in each seed zone to promote diversity and conservation of genetic diversity (Johnson et al. 2018).
In a common garden study in Boise, Idaho (2,779 ft [847 m]), cold hardiness, flowering phenology, and survival were evaluated for five geographically distinct populations representing five provisional seed zones and an elevation range of 2,805 to 6,089 ft (855–1,856 m) (Fisk et al. 2019). This study revealed plasticity in seasonal cold-hardiness but also implications for the selection and movement of sulphur-flower buckwheat seed. Populations that deacclimated or lost their cold tolerance first in the spring came from the warmest and coldest source populations, which also received the least precipitation. This finding suggested early deacclimation was related to drought avoidance. Plants from low-elevation populations showed delayed flowering phenology, and plants from high-elevation populations flowered earliest. Of the early flowering plants, both came from cold source populations, but also the lowest and highest precipitation source populations, suggesting flowering phenology was not related to drought. The lowest survival rates in the garden (75% in yr 1, 54% in yr 11) were for a source population from a site 3,310 ft (1,009 m) above that of the common garden, receiving 1.9 in (4.7 cm) less annual precipitation than the common garden, and experiencing an average annual temperature 7.7 °F (4.3 °C) lower than the common garden. The highest survival rates in the garden (98% in yr 1, 87% in yr 11) were for a source population that was from a site with an elevation and annual temperature nearly identical to the common garden but that received 2.6 in (6.7) cm less in annual precipitation than the common garden. In the common garden, plants were significantly less cold hardy in October 2014 than in October 2013 (P < 0.05), and October 2013 was considerably colder than October 2014 (Fisk et al. 2019).
Because empirical seed zones are not currently available for sulphur-flower buckwheat, generalized provisional seed zones developed by Bower et al. (2014), may be used to select and deploy seed sources. These provisional seed zones identify areas of climatic similarity with comparable winter minimum temperature and aridity (annual heat:moisture index). In Figure 8, Omernik Level III Ecoregions (Omernik 1987) overlay the provisional seeds zones to identify climatically similar but ecologically different areas. For site-specific disturbance regimes and restoration objectives, seed collection locations within a seed zone and ecoregion may be further limited by elevation, soil type, or other factors.
The Western Wildland Environmental Threat Assessment Center’s (USFS WWETAC 2017) Threat and Resource Mapping (TRM) Seed Zone application provides links to interactive mapping features useful for seed collection and deployment planning. The Seedlot Selection Tool (Howe et al. 2017) can also guide restoration planning, seed collection, and seed deployment, particularly when addressing climate change considerations.
Occurrence Map
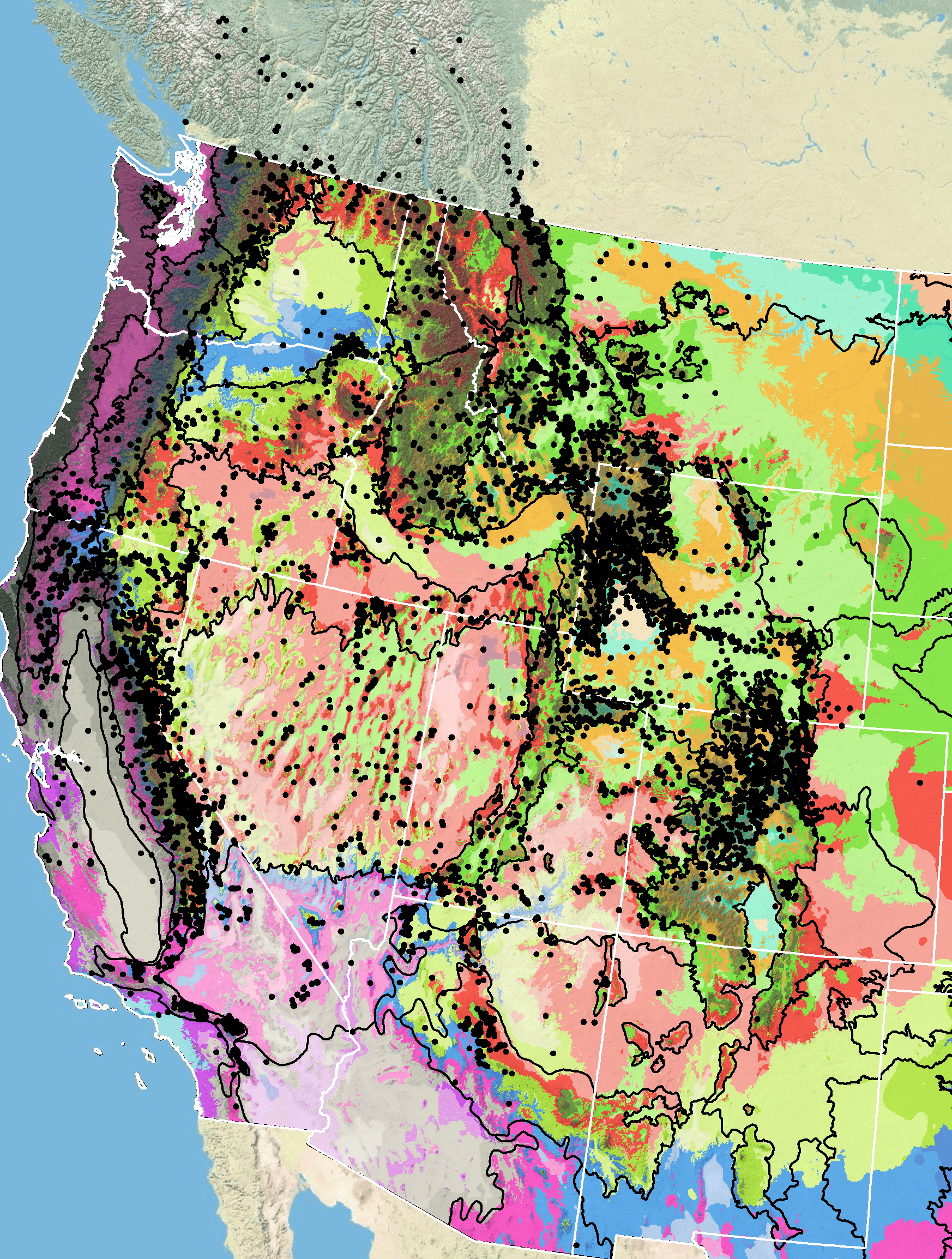
Figure 8. Distribution of sulphur-flower buckwheat (black circles) based on geo-referenced herbarium specimens and observational data from 1861-2016 (CPNWH 2017; SEINet 2017; USDI USGS 2017). Generalized provisional seed zones (colored regions) (Bower et al. 2014) are overlain by Omernik Level III Ecoregions (white outlines) (Omernik 1987; USDI EPA 2018). Interactive maps, legends, and a mobile app are available (USFS WWETAC 2017; www.fs.fed.us/wwetac/threat-map/TRMSeedZoneMapper2.php?). Map prepared by M. Fisk, USDI USGS.
Releases
‘Sierra’ sulphur-flower buckwheat was released in 1987 by the NRCS Plant Materials Center in Lockeford, California, and the California Agricultural Experiment Station (Dyer 2005). The original collection was made in 1972 from 6,200 ft (1,890 m) in elevation in South Lake Tahoe, California. The source material represents the polyanthum variety (Monsen et al. 2004), which is a low-growing shrub. It was developed for stabilization of slopes and use on dry, rocky sites. It does not tolerate wet conditions or poorly drained soils and is recommended for use in Major Land Resource Areas 14, 15, 18, 19, 20, and 22 (Dyer 2005; Young-Matthews 2012).
Wildland Seed Collection
Seed is generally collected from wildland sulphur-flower buckwheat in summer. Location and site characteristics are useful in narrowing down and ultimately distinguishing varieties at any given collection site (see Appendix 1).
Wildland Seed Certification
Verification of species and tracking of geographic source is necessary whether wildland seed is collected for immediate project use or as stock seed for cultivated increase. This official Source Identification process can be accomplished by following procedures established by the Association of Official Seed Certifying Agencies (AOSCA) Pre-Variety Germplasm Program (Young et al. 2020; UCIA 2015). Wildland seed collectors should become acquainted with state certification agency procedures, regulations, and deadlines in the states where they collect.
If wildland-collected seed is to be sold for direct use in ecological restoration projects, collectors must apply for Source-Identified certification prior to making collections. Pre-collection applications, site inspections, and species and seed amount verification are handled by the AOSCA member state agency where seed collections will be made (see listings at AOSCA.org).
If wildland seed collected by a grower or private collector is to be used as stock seed for planting cultivated seed fields or for nursery propagation (See Agricultural Seed Field Certification section), detailed information regarding collection site and collecting procedures must be provided when applying for certification. Photos and herbarium specimens may be required. Germplasm accessions acquired within established protocols of recognized public agencies, however, are normally eligible to enter the certification process as stock seed without routine certification agency site inspections. For contract grow-outs, however, this collection site information must be provided to the grower to enable certification.
Collection Timing
Wildland seed is generally ready for harvest from June to September although it may be ready earlier or later depending on elevation and weather. Seed is typically mature when flower parts are dry and papery and, in some cases, have turned tan or red-orange in color (Fig. 9) (Parkinson and DeBolt 2005; Luna and Corey 2008; Blanke and Woodruff 2011; Young-Matthews 2012). Mature seeds are hard and cannot be crushed by thumbnail pressure (Young 1989). Typically seeds are retained on the plant for up to 3 weeks once mature (Young-Matthews 2012), but Parkinson and DeBolt (2005) recommend periodic checks to maximize harvests. Although Monsen et al. (2004) indicated sulphur-flower buckwheat produced good seed crops in most years, even dry years, Young (1989) reported low or no seed production in several years over a 15-year period.

Figure 9. Papery sulphur-flower buckwheat flowers signaling mature seed is available at this site in Colorado. Photo: USDI BLM CO932, SOS.
Collection Methods
Wildland seed is typically collected by hand stripping or clipping seed heads (Fig. 10), rubbing dried seed heads together, or beating seed heads into a container (Young and Young 1986; Rose et al. 1998; Monsen et al. 2004; Parkinson and DeBolt 2005). Holmgren (1954) reported sulphur-flower buckwheat seeds that remained on the plant for a considerable time after ripening and were easily collected by hand stripping.

Figure 10. Hand stripping seed from E. u. var. nevadense in California. Photo: USDI BLM CA170, SOS.
Several collection guidelines and methods should be followed to maximize the genetic diversity of wildland collections: 1) collect seed from a minimum of 50 randomly selected plants; 2) collect from widely separated individuals throughout a population without favoring the most robust or avoiding small stature plants; and 3) collect from all microsites including habitat edges (Basey et al. 2015). General collecting recommendations and guidelines are provided in online manuals (e.g., ENSCONET 2009; USDI BLM SOS 2021).
It is critical that wildland seed collection does not impact the sustainability of native plant populations. Collectors should take no more than 20% of the viable seed available at the time of harvest (USDI BLM SOS 2021). Additionally, care must be taken to avoid the inadvertent collection of weedy species, particularly those that produce seeds similar in shape and size to those of sulphur-flower buckwheat.
Post-Collection Management
Sulphur-flower buckwheat flowers and stems are typically quite dry at the time of harvest, but bracts and flower material can absorb moisture at night or at other times when humidity is high. Harvested seed should be thoroughly dried and then treated to 48 hours of freezing or with an insecticide to kill insect pests prior to storing (Fig. 11) (Parkinson and DeBolt 2005).

Figure 11. Seed collection gathered by clipping seed heads from E. u. var. majus in Wyoming. Photo: USDI BLM WY030, SOS.
At the J Herbert Stone Nursery (JHSN), harvested seed was put in drying bins (4 × 4 × 1.5 ft [1.2 × 1.2 × 0.5 m]) with fine mesh screens, which allowed air circulation. Bins were stacked six high, and warm air was blown up through the bins. After 12 hours, if the seed moisture content was less than 8%, seeds were packed into plastic bags. The plastic bags were put into boxes and stored at 0 to 36 °F (0–2 °C). This post-collection management and storage resulted in many years of seed viability (Archibald 2006).
Seed Cleaning
Difficulty of seed cleaning varies by seed lot and collection method. Collections with a lot of plant material or large amounts of unfilled seed are more difficult to clean because unfilled seeds and flower bracts can be difficult to remove (Stevens et al. 1996; Wall and MacDonald 2009). It is common for collections to include perianths, involucres, and inflorescence branches with the seed. Because the ovule within the seed is anatropous, the radicle end is pointing outward and upward making germination possible with the perianth still attached (Meyer 2008). Depending on the amount of plant material in the collection, seed is generally cleaned satisfactorily through a hammermill, barley de-bearder, or gentle brush machine to break the seeds from the stalks and bracts followed by passing the collection through an air-screen machine to remove chaff and weed seeds (Monsen et al. 2004; Young-Matthews 2012). Material should not be handled too roughly because the radicle end of the achene is typically slender and easily damaged (Fig. 12) (Meyer 2008).

Figure 12. Clean sulphur-flower buckwheat seed processed by Bend Seed Extractory. Photo: USFS.
For small seed lots, Parkinson and DeBolt (2005) first processed seed heads on a rubbing board while hand removing larger debris. Seeds were then processed through a 4.7 mm sieve (# 4), followed by a 1 mm sieve (# 18), then four times through a seed blower (setting 30), and a final pass over a 2.3 mm sieve (# 8). This process yielded 120,000 to 200,000 seeds/lb (265,000-441,000/kg). Wall and MacDonald (2009) rubbed floral material over #14 and #25 sieves with a blower speed of 1.75. Hand sorting was required because the seed lot had a lot of empty seed. Difficulty of the cleaning process was rated a 4, where 5 was most difficult, and the seed lot required 6 to 10 hours to process (Wall and MacDonald 2009).
Several facilities reported seed cleaning procedures for wildland-collected seed. At the Upper Colorado Environmental Plant Center, wildland-collected seed was first processed using a Forsberg gravity table (Thief River Falls, Minnesota), then run through a Clipper air cleaner with a # 7 screen on top and 1/24 screen on the bottom. Air speed was minimal to low and increased gradually as needed (Blanke and Woodruff 2011). At the Glacier National Park Nursery, wildland-collected seed was cleaned using an air blower with screens to separate chaff from live seeds. This procedure yielded 218,000 seeds/lb (480,000 seeds/kg) with 100% purity and 89% germination (Luna and Corey 2008). At the Bend Seed Extractory, a small hand-collected seed lot (6.3 lbs [2.9 kg]) was first processed using a Westrup Model LA-H laboratory brush machine (Slagelse, Denmark) with a #20 mantel and speed setting of 3 to remove the seed from the seed heads. Seeds were finished by air-screening to remove the remaining nonviable seed and inert material, using an Office Clipper with #7 top and 1/12 round bottom screen followed by round bottom screens of 1/21 to 1/25 with medium speed and medium to high air. This procedure yielded 217,000 seeds/lb (478,000 seeds/kg) with 96% purity, 96% fill, and 96% viability (Barner 2009b).
At the Oregon State University Malheur Experiment Station, unthreshed sulphur-flower buckwheat seed harvested using a small-plot combine was manually processed through a meat grinder and then through a small clipper (Shock et al. 2017).
At the Bend Seed Extractory the following procedure was used for cleaning a large nursery-grown seed lot (294 lbs [133 kg]). The procedure resulted in 168,000 seeds/lb (370,000 seeds/kg) with 99.6% purity, 97% fill, 83% viability (TZ), and moisture content was 9.2% (Barner 2009a) –
- Process using a Westrup Model HA 400 brush machine (Slagelse, Denmark) with a speed of 3 to remove seed from seed head;
- Air screen using a Clipper Eclipse Model 324 (Corvallis, Oregon) with #6 round top screen to remove chaff and inert material;
- Screen again using #6 round top and 1/25 bottom screen with medium to high air;
- Finish using an Oliver Model 30 Gravity Separator (Rocky Ford, Colorado) with speed 20, air 50, and hopper speed 1.5.
Seed Storage
Sulphur-flower buckwheat seed collections should be treated with an insecticide or by freezing to kill any insects. Seed should be stored in rodent-proof containers (Stevens et al. 1996). Dry seed (<15% moisture content) (Parris et al. 2010) retains viability longer at cool or cold rather than room temperature storage conditions (Kay et al. 1988). Clean seed processed by the Bend Seed Extractory was stored at 33 to 38 °F (0.6–3 °C) (Barner 2009a).
Reports on the viability retention of stored sulphur-flower buckwheat seed varied from a few to 15 years (Stevens et al. 1996; Dyer 2005). Wildland seed collected in Ada County, Idaho, germinated at 86% after 18 months of storage in a brown glass bottle kept at room temperature for 18 months (Parkinson and DeBolt 2005). Seed from production fields grown at the Lockeford, California, Plant Materials Center lost viability rapidly within a few years (Dyer 2005). Wildland seed collected in Mono County, California, retained germinability better when stored at cool 39 °F (4 °C) or cold 5 °F (-15 °C) temperatures than when stored at room or warehouse temperatures. At any storage temperature, though, germinability of seed decreased after 5 years. Seed also kept better when stored in sealed jars rather than in cloth or paper bags (Kay et al. 1988). At Glacier National Park Nursery, Montana, sulphur-flower buckwheat seed retained viability up to 7 years in sealed containers stored at 37 to 41 °F (3–5 °C) with low relative humidity (Luna and Corey 2008). Stevens et al. (1996) reported sulphur-flower buckwheat seed longevity of 10 to 15 years.
Seed Testing
To test for germination, seeds can be prechilled at 37 to 41 °F (3–5 °C), then incubated at 59 to 77 °F (15–25 °C). The first germination count should occur after 5 days and the final count at 28 days (Chirco and Turner 1986). The AOSA tetrazolium chloride viability procedure for Eriogonum species recommends a 45° angle cut, removal of the distal end, and a 1% TZ concentration soak overnight at 86 to 95 °F (30–35 °C). Seed is non-viable if any part of the embyo is unstained (AOSA 2010).
Germination Biology
Sulphur-flower buckwheat seed requires at least a 30- to 40-day afterrippening period to reach maximum germinability (Stevens et al. 1996; RBG Kew 2019). At least a portion of the seed will germinate without pretreatment (Young and Young 1986), but the rate of germination can be improved if seeds are cold stratified (Dyer 2005).
An afterripening period improves germination of sulphur-flower buckwheat. For seed collected from 9 populations, germination percentages were significantly different across all sources (P < 0.001), and germination increased with after-ripening periods of 4 to 5 years in dry cold storage (Davis et al. 2014). Wildland-collected seed stored for 18 months at room temperature germinated at 86% without any pretreatment. Seed was put on moist blotter paper in a germination chamber (constant 72 °F [22 °C], 12 hrs light/12 hrs dark). Germination started within 4 days, continued sporadically for 60 days, and was 70% within 48 days (Parkinson and DeBolt 2005).
Testing done on sulphur-flower buckwheat (var. umbellatum) seed collected from the eastern Sierra Nevada Mountains in Nevada, revealed variable annual seed production and germination potential and improved germination with large temperature fluctuations and exposure to warm temperatures (Table 4; Young 1989). Mature seed was collected in 9 of 15 years between 1974 and 1988, because seed production was low to none in the other 6 years. Germination reached a maximum of 90% in some years and just 50% in other years. Non-germinating seeds were often empty or contained shrunken embryos. In evaluations of fluctuating temperature effects on germination, larger temperature fluctuations with warm temperature exposure resulted in the best germination. Seed germination was evaluated in dark incubators with 16 hours of light and 8 hours of dark (Table 4; Young 1989).
Table 4. Germination of sulphur-flower buckwheat at various fluctuating temperatures (Young 1989).
|
Seedbed temperature |
Temperature (°F) range (8/16 hr exposure) |
Average germination (%) |
|
Very cold |
(32–36/32–41) |
20c |
|
Cold |
(32–41/41–59) |
41b |
|
Cold Fluctuating |
(32–36/68–104) |
65a |
|
Fluctuating |
(41–59/86–104) |
75a |
|
Moderate |
(41–77/50–95) |
64a |
|
Warm |
(68–104/86–104) |
39b |
Germination percentages followed by different letters are significantly different (P <0.01).
Although a portion of sulphur-flower buckwheat seed lots germinate without pretreatment, cold temperature exposure, the primary regulator of dormancy status for Eriogonum species, can increase percent germination (Meyer and Paulsen 2000). Kramer and Foxx (2016) found that cold temperature exposure (12 wks at 34 °F [1 °C]) improved germination (≥ 75%) of sulphur-flower buckwheat seed (source identified Uncompahgre Partnership). Germination was poor (≤ 5%) for seed not exposed to cold and germinated at alternating day/night temperatures of 52 to 68 °F/34 to 50 °F (11–20 °C/1–10 °C).
For sulphur-flower buckwheat populations in Utah representing three varieties, germination was low (<20%) without chilling, but 12 or more weeks of chilling resulted in complete dormancy removal (Table 5; Meyer and Paulsen 2000). Seed was collected from at least 20 plants at each of five Utah locations. Seed was stored in paper envelopes in a laboratory (68–72 °F [20–22 °C], 30–40% RH) for 1 to 2 months before testing. Variety porteri seed collected from an alpine tundra community was most dormant, requiring 24 weeks of storage to completely lose dormancy. Across all seed collected for the Eriogonum genus, collections from high-elevation habitats with long winters required longer incubation to germinate under cold conditions than collections from warm, low-elevation habitats (Table 5; Meyer and Paulsen 2000).
Table 5. Variability in seed size, germination, and viability for sulphur-flower buckwheat varieties growing at increasing elevations in Utah (Meyer and Paulsen 2000).
|
Variety |
Collection site County (elevation [ft]) |
Date |
100 seed weight (g) |
Highest germination at various durations of chilling at 36 °F |
Mean viability (%) |
|
umbellatum |
Sevier (7,050) |
Aug 26 |
1.4 |
98% after 12 wks; 100% after 16 wks |
88 |
|
umbellatum |
Wayne (8,370) |
Aug 26 |
1.9 |
97% after 12 wks; 100% after 16 wks |
85 |
|
umbellatum |
Garfield (7,640) |
Aug 27 |
2.6 |
98% after 8 wks; 100% after 12 wks |
90 |
|
majus |
Utah (7,450) |
Aug 8 |
2.1 |
92% after 12 wks; 100% after 16 wks |
81 |
|
porteri |
Summit (11,420) |
Sept 7 |
2.6 |
45% after 16 wks; 94% after 24 wks |
78 |
In experiments to evaluate smoke cues for seed germination, a smoke dilution of 1:10 significantly reduced sulphur-flower buckwheat germination relative to controls. Smoke diluted with distilled water was used to moisten germination papers. Smoke dilutions of 1:100 and 1:1,000 did not affect germination (Cox 2016).
Wildland Seed Yield And Quality
Post-cleaning seed yield and quality of seed lots collected in the Intermountain region are provided in Table 6 (USFS BSE 2017). The results indicate that sulphur-flower buckwheat seed weight is variable. Seed fill and viability are also variable, but these can generally be cleaned to high levels. The numbers of seeds/lb (120,000–208,695) reported by others (Stevens et al. 1996; Dyer 2005; Parris et al. 2010; Wiese et al. 2012; USFS GBNPP 2014) fell within the range provided in Table 6.
Table 6. Seed yield and quality of sulphur-flower buckwheat seed lots collected in the Intermountain region, cleaned by the Bend Seed Extractory, and tested by the Oregon State Seed Laboratory or the USFS National Seed Laboratory (USFS BSE 2017).
|
Seed lot characteristic |
Mean |
Range |
Samples (no.) |
|
Bulk weight (lbs) |
8.65 |
0.13–260 |
127 |
|
Clean weight (lbs) |
1.17 |
0.001–42 |
127 |
|
Clean-out ratio |
0.13 |
0.006–0.56 |
127 |
|
Purity (%) |
96 |
80–99 |
127 |
|
Fill (%)¹ |
89 |
13–99 |
127 |
|
Viability (%)² |
82 |
35–98 |
96 |
|
Seeds/lb |
201,135 |
101,000–453,600 |
127 |
|
Pure live seeds/lb |
153,827 |
68,632–250,179 |
96 |
¹100 seed X-ray test
²Tetrazolium chloride test
Marketing Standards
Acceptable seed purity, viability, and germination specifications vary with revegetation plans. Purity needs are highest for precision seeding equipment used in nurseries, while some rangeland seeding equipment handles less clean seed quite well.
Stevens et al. (1996) indicated that viability can exceed 90% when sulphur-flower buckwheat seeds are mature. Seed can be cleaned to 95 to 98% purity, but immature seeds and flower bracts may be difficult to remove.
Agricultural Seed Production
Seed production of sulphur-flower buckwheat has been evaluated by researchers at Oregon State University’s Malheur Experiment Station (OSU MES) (Shock et al. 2017), J Herbert Stone Nursery (JHSN) (Archibald 2006), and Aberdeen Idaho Plant Materials Center (PMC) (St. John and Ogle 2011). Their management practices and relationships to successful seed production are provided in the sections below. At OSU MES (Fig. 13), sulphur-flower buckwheat produced seed the second year after seeding and crops were harvested for 11 years (Shock et al. 2017).
Figure 13. Sulphur-flower buckwheat seed production plot growing at OSU MES in Ontario, OR. Photo: C. Shock, OSU MES.
Agricultural Seed Certification
In order to minimize genetic changes in specific accessions of native species when increased in cultivated fields, it is essential to track the geographic source and prevent inadvertent hybridization or selection pressure. This is accomplished by following third party seed certification protocols for Pre-Variety Germplasm (PVG) as established by the Association of Official Seed Certification Agencies (AOSCA). AOSCA members in the U.S., Canada, and other countries administer PVG requirements and standards that track the source and generation of planting stock. Field and cleaning facility inspections then monitor stand establishment, proper isolation distances, control of prohibited weeds, seed harvesting, cleaning, sampling, testing, and labeling for commercial sales (Young et al. 2020; UCIA 2015).
Seed growers apply for certification of their production fields prior to planting and plant only certified stock seed of an allowed generation (usually less than four). The systematic and sequential tracking through the certification process requires preplanning, knowing state regulations and deadlines, and is most smoothly navigated by working closely with state certification agency personnel. See the Wildland Seed Certification section for more information on stock seed sourcing.
Site Preparation
A weed-free, smooth, level, firm seedbed is recommended for accurate shallow seeding (Parris et al. 2010; Blanke and Woodruff 2011; Young-Matthews 2012). Sulphur-flower buckwheat grows well in moderate-textured soils with good drainage and slightly basic to neutral pH (Stevens et al. 1996). At the JHSN in southwestern Oregon, fields were fumigated, then ripped and disked into raised beds, then broadcast fertilized with ammonium phosphate and potassium sulfate (250 lbs/ac of each) before seeding (Archibald 2006).
Seed Pretreatments
Planting cold-stratified seed in early March at OSU MES in Ontario, Oregon, when fall seeding was not possible, resulted in only sparse stand establishment (Shock et al. 2017).
Weed Management
A combination of mechanical and chemical treatments were used to control weeds in seed production fields at the Aberdeen PMC and OSU MES. At the Aberdeen PMC, weeds were controlled using a combination of weed barrier fabric, spring herbicide treatments of 100% glyphosate applied using the wick method, and hand weeding about 4 weeks after herbicide treatments (St. John and Ogle 2011). At OSU MES, Shock et al. (2018) applied post-emergent herbicides annually (various chemicals) and hand weeded stands as needed. At JHSN, weeds were controlled by mowing and cultivating between the rows and by hand within the bed, which was effective but costly (Archibald 2006). At Corvallis, Oregon PMC, weeds were controlled through a combination of between-row tillage, hand removal, and targeted herbicide treatments (Young-Matthews 2012).
There are no herbicides labeled for use on sulphur-flower buckwheat seed crops (Parris et al. 2010), but various pre-emergent and post-emergent herbicides were tested in research studies at OSU MES. Pre-emergent herbicides were applied to plots on November 30, 2009, prior to seeding sulphur-flower buckwheat on December 1, 2009. Emergence was poor and uneven, and just 37% of seed emerged in untreated rows. Emergence was significantly lower with pre-emergent treatments of pronamide (0.7%) and trifluralin (12%) (P < 0.05). Emergence was also lower, although not significantly so, with pre-emergent treatments of pendimethalin, benefin, acetamide, and linuron (13-19% emergence). Emergence with pre-emergent bensulide treatments and pendimethalin treatments with activated charcoal (35–39%) were nearly the same as from untreated plots (Shock et al. 2011).
Annual applications of post-emergent herbicides were also tested in research studies at OSU MES. In these trials sulphur-flower buckwheat was seeded on November 1, 2005 and treatments were made May 24, 2006; April 24, 2007; March 13, 2008; March 20, 2009; and April 7, 2010. Seed yield was reduced least or slightly improved with post-emergent treatments of pendimethalin (0.95–1.19 lbs ai/ac [1.06–1.33 kg ai/ha]) and acetamide (0.66–0.98 lbs ai/ac [0.73–1.10 kg ai/ha]) treatments, which are soil active herbicides. Most of the other treatments, all foliar active herbicides, resulted in lower seed yield than the weed-free control (Shock et al. 2011).
Table 7. Seed yield for sulphur-flower buckwheat treated annually with various post-emergent herbicides in seed production plots growing at Oregon State University’s Malheur Experiment Station in Ontario, OR (Shock et al. 2011).
|
Treatment |
2007 |
2008 |
2010 |
|
Clean seed yield* (lbs/ac) |
|||
|
Weed free, Untreated |
91.7a |
365.1a |
831.1a |
|
Bromoxynil |
38.1b |
285.5a |
354.5b |
|
Oxyfluorfen |
42.6a |
279.7a |
271.5b |
|
Clethodim |
57.6a |
263.0a |
625.6a |
|
Pendimethalin |
115.0a |
385.0a |
773.7a |
|
Prometryn |
27.3b |
298.5a |
470.5b |
|
Acetamide |
75.1a |
354.8a |
853.5a |
|
Linuron |
35.6b |
368.4a |
460.7a |
Values within a column with different letters are significantly different (P < 0.05). Seed was harvested by hand, and severe root rot resulted in very little seed production in 2009.
Seeding
Although rates and methods for seeding varied, dormant seeding in the fall or winter at shallow depths was the practice at all seed production fields. Seeding rates ranged from 2.25 to 12 lbs PLS/ac (2.5–13 kg PLS/ha) (Parris et al. 2010; Young-Matthews 2012) or 15 to 33 PLS/ft (50–109 PLS/m) (Stevens et al. 1996; Blanke and Woodruff 2011). Seeds were hand planted, belt-seeded, or drill-seeded into rows or hills (Stevens et al. 1996; Parris et al. 2010).
General seeding recommendations from Stevens et al. (1996) were to plant sulphur-flower buckwheat seeds on the surface of disturbed soil or up to 0.25 in deep by hand or using a single-row seeder. The recommended seeding rate was 15 to 20 PLS/linear ft (50–66 PLS/m) of row or 4 to 5 PLS in hills spaced 2 to 3 ft (0.6-0.9 m) apart. Recommended row and plant spacing (if using transplants) was 30 to 36 in (76–91 cm) (Stevens et al. 1996).
At the Corvallis PMC, seed was planted 0.25 in deep in the fall at a rate of 10 to 12 lbs/ac (11.2–13.4 kg/ha) in rows 18 to 24 in (46–61 cm) apart (Young-Matthews 2012). At the Upper Colorado Environmental Plant Center (UCEPC), clean untreated seed was planted in August at a 0.25-in depth at a rate of 30 to 33 PLS/ft (99–109 PLS/m) using a belt seeder (Blanke and Woodruff 2011). At JHSN, seeding was done in the fall. Seed was planted in four bands (0.7 in [1.9 cm] deep, 1.2 in [3 cm] wide, and 11.2 in [30 cm] apart) using a modified Love/Oyjord seed drill (Garfield, Washington) with packing wheels that pressed seeds into the soil. Bands were covered with 6 to 8 mm layers of sawdust and kept moist until fall rains began (Archibald 2006). At the Aberdeen PMC, sulphur-flower buckwheat was direct dormant seeded in November 2005. By June 2007, survival of emerged seedlings was 40% following targeted weed control measures (St. John and Ogle 2011). At OSU MES, cold-stratified seed was planted in March. Seed was planted 0.5 in (1.3 cm) deep at 20 to 30 seeds/ft (65–100 seeeds/m) of row using a custom, small-plot, grain drill. This produced thin stands, so empty row sections were re-seeded in late October and by the following spring there were 3 plants/ft (10 plants/m) (Fig. 13 and 14; Shock et al. 2017).
Establishment And Growth
Sulphur-flower buckwheat was considered established 2 years after seeding at the UCEPC. Growth was rapid in the warm summer months, and plots were protected from predation by a snow fence (Blanke and Woodruff 2011). Most crop producers fertilized sulphur-flower buckwheat stands, yet over fertilization was cautioned against, and applications in mid-spring to early summer every 2 years were considered sufficient (Stevens et al. 1996). At OSU MES, Shock et al. (2018) fertilized 1-year-old stands with phosphorus (50 lbs/ac [56 kg/ha]) and zinc (2 lbs/ac [2.2 kg/ha]) and did not fertilize again. At JHSN, fields were broadcast fertilized with a mix of ammonium phosphate and potassium phosphate (250 lb/ac [280 kg/ha] each) before seeding. Seedlings grew slowly (2–3 in [5–8 cm]) through the winter, but experienced rapid vegetative growth in March and early April and flowered in May. Plants were fertilized and irrigated frequently at the time of rapid vegetative growth and again when flowering with ammonium nitrate (100 lbs/ac [112 kg/ha] each time). Established older plantings were fertilized once in early spring (250–300 lbs/ac [280–336 kg/ha] NPK 13-13-13) (Archibald 2006).

Figure 14. Sulphur-flower buckwheat seedlings emerging in seed production plots at OSU MES. Photo: C. Shock, OSU MES.
Irrigation
Sulphur-flower buckwheat requires minimal irrigation and several crop producers irrigated stands only until established. Plants are susceptible to root and crown rot with too much water. Even mature stands may weaken with over irrigation (Young-Matthews 2012; Stevens et al. 1996). Stevens et al. (1996) recommended irrigation as needed until plants were established and indicated that plants grew well with 12 to 16 in (305–406 mm) of annual precipitation. At JHSN, establishing fields were kept moist until fall rains began, and harvested fields were irrigated in early fall to encourage root growth (Archibald 2006). At the Corvallis PMC, irrigation was sometimes provided in the first year, but rarely, if at all, after plants were established (Young-Matthews 2012).
In a test of field growth with and without irrigation (Figs. 8-10), total dry matter production of sulphur-flower buckwheat was not significantly different between irrigated (9.9 gal [43.7 l] water/plant added) and non-irrigated (2.8 in [70 mm] rainfall over 11 weeks) plants growing near Fort Collins, Colorado. Increases in height and width, however, were significantly greater for sulphur-flower buckwheat in non-irrigated than in irrigated sections of the field (Cox and Klett 1984).
Table 8. Flowering and harvest timing as related to timing of irrigation of sulphur-flower buckwheat seed production plots growing at Oregon State University’s Malheur Experiment Station in Ontario, OR (Shock et al. 2017)
|
Year |
Flowering |
Irrigation |
Harvest |
||
|
Start |
End |
Start |
End |
||
|
2006 |
19 May |
20 Jul |
19 May |
30 Jun |
3 Aug |
|
2007 |
25 May |
25 Jul |
2 May |
24 Jun |
31 Jul |
|
2008 |
5 Jun |
20 Jul |
15 May |
24 Jun |
24 Jul |
|
2009 |
31 May |
15 Jul |
19 May |
24 Jun |
28 Jul |
|
2010 |
4 Jun |
15 Jul |
28 May |
8 Jul |
27 Jul |
|
2011 |
8 Jun |
20 Jul |
20 May |
5 Jul |
1 Aug |
|
2012 |
30 May |
4 Jul |
30 May |
11 Jul |
24 Jul |
|
2013 |
8 May |
27 Jun |
8 May |
19 Jun |
9 Jul |
|
2014 |
20 May |
1 July |
13 May |
24 Jun |
10 Jul |
|
2015 |
13 May |
25 Jun |
29 Apr |
10 Jun |
2 Jul |
|
2016 |
16 May |
25 Jun |
27 Apr |
7 Jun |
1 Jul |
Sulphur-flower buckwheat seed production was increased with irrigation in dry years at OSU MES in Ontario, Oregon (annual precipitation: 10 in [254 mm]). Seed yield and plant water relations were evaluated at OSU MES over 11 years (2006-2016). Studies tested additions of 0, 4, and 8 in (0, 100, 200 mm) of underground drip irrigation delivered 12 in (30 cm) deep in four bi-weekly increments starting at time of flower bud formation (Table 8). Over the course of the study, annual precipitation averaged 10 in (257 mm) (range: 5.3–14.5 in [135–368 mm]). Seed yield varied over years and was not related to growing degree days. Highest seed yield by treatment averaged across years was 232 lbs/ac (260 kg/ha) (range: 185–453 lbs/ac [207-508 kg/ha]) (Table 9). Seed yield had no significant response to irrigation in 2010, a wet year and declined with irrigation in 2011, the year with the highest rainfall (Table 10). The positive response to irrigation in 2006, also a wet year, may have been because it was the first year of production and plants were smaller (Shock et al. 2017). Irrigation was delivered by a subsurface drip system, which allows for irrigation automation, decreases weed pressure, and leaves good field access, but has drawbacks in startup and maintenance costs (Parris et al. 2010).
Table 9. Maximum seed yield of sulphur-flower buckwheat seed production plots by irrigation amount applied based on quadratic response. Plots at Oregon State University’s Malheur Experiment Station in Ontario, OR (Shock et al. 2017).
|
Year |
Highest yield (lbs/ac) |
Fall + winter + spring ppt (in) |
Growing hrs (50-86 °F) Jan-Jun |
Water (in) added for highest seed yield |
|
2006 |
32.8 |
14.5 |
707 |
8.0 |
|
2007 |
193.7 |
6.2 |
781 |
7.9 |
|
2008 |
246.5 |
6.7 |
604 |
7.1 |
|
2009 |
242.5 |
8.9 |
671 |
6.8 |
|
2010 |
264.2 |
11.7 |
539 |
2.5 |
|
2011 |
232.5 |
14.4 |
476 |
0.0 |
|
2012 |
185.5 |
8.4 |
682 |
8.0 |
|
2013 |
396.2 |
5.3 |
733 |
5.6 |
|
2014 |
453.4 |
8.1 |
741 |
5.2 |
|
2015 |
199.3 |
10.4 |
895 |
3.4 |
|
2016 |
233.3 |
10.1 |
810 |
3.3 |
|
Mean |
231.7 |
9.1* |
665** |
5.3 |
Seed harvested using small-plot combine in all yrs but 2013 and 2016 when harvested by hand.*72-yr average; **23-yr average.
Table 10. Seed yield (lbs/ac) for sulphur-flower buckwheat in response to no additional irrigation and supplemental irrigation (4 and 8 in [100 and 200 mm]) at Oregon State University’s Malheur Experiment Station in Ontario, OR (Shock et al. 2017)
|
Year |
Supplemental irrigation (in/season*) |
||
|
0 |
4 |
8 |
|
|
———Seed yield (lbs/ac)——— |
|||
|
2006 |
155.2a** |
214.3a |
333.2b |
|
2007 |
79.5a |
164.6b |
193.7b |
|
2008 |
121.3a |
221.4b |
245.0b |
|
2009 |
132.2a |
222.9b |
240.0b |
|
2010 |
252.7a |
260.1a |
208.6a |
|
2011 |
248.5b |
136.9a |
120.9a |
|
2012 |
61.2a |
153.1b |
185.3b |
|
2013 |
113.2a |
230.0b |
219.7b |
|
2014 |
256.9a |
441.5b |
402.5b |
|
2015 |
136.4a |
197.7a |
90.6a |
|
2016 |
183.3a |
231.7a |
140.7a |
|
Average |
158.2a |
215.8b |
219.9b |
*Irrigation through a drip system applied from bud to seed set.**Numbers within a row with different letters are significantly different (P < 0.05). Seed harvested using small-plot combine in all yrs but 2013 and 2016 when harvested by hand
Pollinator Management
Bee pollination can increase yield of sulphur-flower buckwheat seed, and the suggested stocking density is one strong honey bee hive per acre (Cane cited in Parris et al. 2010).
Pest Management
Seed loss from insects can range from 10 to 35%, and no control measures have been developed (Stevens et al. 1996). At JHSN no insect or disease problems were reported (Archibald 2006). Few pest or disease issues were reported on sulphur-flower buckwheat crops at OSU MES, but rust infections may potentially require control, and too much irrigation can result in secondary infection or disease problems (Parris et al. 2010). Sulphur-flower buckwheat is a host for Gloeosporium eriogoni and Uromyces intricatus fungi (Farr and Rossman 2017), and Leveillula taurica and Uromyces spp. fungi were found on sulphur-flower buckwheat plants in Idaho and Oregon (Sampangi et al. 2012).
Seed Harvesting
Sulphur-flower buckwheat seed is produced in harvestable quantities when plants are 2 years old or older (Stevens et al. 1996; Young-Matthews 2012). Seed typically ripens by mid to late summer, and mature seed persists on plants for 1 to 3 weeks (Parris et al. 2010). Seed can be harvested mechanically by modified combining, mechanical stripping, vacuuming, or non-mechanically using rice knives, beating seed heads into a container, or stripping the seed heads by hand (Stevens et al. 1996; Young-Matthews 2012). Plants can be pruned back after flowering to promote a denser, more compact plant (Young-Matthews 2012).
Seed is often ready for harvest in summer but exact timing depends on latitude, elevation, local weather, and plant variety. Seed may ripen by July 1 when grown at lower elevations (≤5,000 ft [1,500 m]) or as late as mid-August or September when grown at higher elevations (Stevens et al. 1996; Parris et al. 2010). Seeds are mature when petals and sepals (perianths) are dry and papery (Young-Matthews 2012). At JHSN, fields are monitored weekly for seed ripeness and then daily as maturation occurs (Archibald 2006).
Several crop producers combined mechanical and hand harvesting methods to maximize yields (Archibald 2006; St. John and Ogle 2011; Young-Matthews 2012). The fairly uniform seed ripening and mature seed retention of 1 to 3 weeks allows for the use of standard combine methods (Parris et al. 2010). At Aberdeen PMC, sulphur-flower buckwheat seed was harvested first using a combine then by hand (St. John and Ogle 2011). At JHSN, seed was hand collected first and later using a small-plot combine (Archibald 2006). At OSU MES, seed was harvested using a small-plot combine in all but 2 of 11 years (Table 10; Shock et al. 2017). At the Lockeford (California) PMC, seed was harvested by hand or with a vacuum (Dyer 2005). Although several crop producers used combines to harvest sulphur-flower buckwheat seed, Young-Matthews (2012) suggested seed can be easily damaged by threshers or combines and recommended large plots be harvested using a seed stripper (Tilley and Bair 2011). At the Aberdeen PMC, staff developed a tool called the Jet Harvester that vacuums ripe seed. In one hour the Jet Harvester collected the same amount that two people did by hand in 2 days. The Jet Harvester also reduces the time spent cleaning seed lots. Instructions on building this tool are provided (Tilley and Bair 2011).
Seed Yields And Stand Life
Sulphur-flower buckwheat seed production is 30 to 50% of full production by the second post-seeding year and 100% after that if stands remain relatively disease and insect free (Stevens et al. 1996). Seed harvest yields range from 150 to 700 lbs/ac (168–785 kg/ha) and stands can produce harvestable seed for up to 20 years if plant loss is low and recruitment is controlled to avoid certification problems (Young-Matthews 2012). Stevens et al. (1996) indicated that seed yields of 200 to 400 lbs/ac (224–448 kg/ha) at 95% purity were typical. Seed fields grown at the Lockeford PMC averaged 150 lbs seed/ac (168 kg/ha) (Dyer 2005).
Nursery Practice
Several nurseries reported their propagation and growing procedures for producing sulphur-flower buckwheat container stock. Seeds planted outdoors in the fall required no pretreatment (Rose et al. 1998), but seeds planted indoors were often cool-moist stratified for between 2 weeks and 3 months (Everett 1957). Various growth media mixes were used successfully and damping off was a common cause of mortality.
The Rocky Mountain Research Station in Boise, Idaho, used the following procedure to produce sulphur-flower buckwheat plugs. Seeds were germinated on moist blotter paper and developed true leaves about 16 days following germination. Germinants were planted 0.4 in (1 cm) deep in cone-tainers filled with equal parts peat and vermiculite. Cone-tainers were placed in the greenhouse (constant 81°F [27 °C]), automatically watered once saturation fell below 80%, and fertilized periodically. Seedling survival was 52%. Damping off was the major cause of seedling mortality (Parkinson and DeBolt 2005).
At Glacier National Park Nursery, it took 6 months to produce sulphur-flower buckwheat cone-tainer plugs that were 1 cm tall with multiple leaves and a firm root system. Seeds were soaked in water for 10 minutes before 60 to 90 days of cold moist stratification (seeds placed in mesh bags, buried in peat moss, and refrigerated at (34–37 ° F [1–3 °C]). Germinants and stratified seeds without radicle emergence were planted in 6:1:1 peat:perlite:vermiculite, lightly covered with perlite, and thoroughly watered. Cone-tainers were kept in a greenhouse (12 hrs of light at 70–77 °F [21–25 °C] and 12 hrs of dark at 61–64 °F [16–18 °C]). Germination was not uniform and occurred over a period of 3 weeks. Cotelydons developed within 10 days of sowing, and true leaves within 3 weeks. Irrigation occurred only when cone-tainers were dry on the surface, and it was important to let seedlings dry between watering. Plants were considered established after 6 weeks, and at 8 weeks had multiple leaves and plantlets. Seedlings were fertilized twice a week (NPK 10-20-20 liquid 100 ppm). At 12 weeks, plants were moved to an outdoor nursery and were fully root tight at 18 weeks. Plants were fertilized in September (10-20-20 NPK 200 ppm) when irrigation was reduced. Plants overwintered in the outdoor nursery under insulating foam cover and snow (Luna and Corey 2008).
The following procedure was used to grow sulphur-flower buckwheat plants for nursery studies. Seed was cold moist stratified (39 °F [4 °C]) for 2 weeks, then germinated in a growth chamber (75 °F [24 °C], 14 hrs light/10 hrs dark). Germination occurred within 5 to 8 days. Germinants were planted in containers filled with 1:1:1 silt loam:washed concrete:peat moss and put in the greenhouse (64–75 °F [18–24 °C], 14 hrs light/10 hrs dark). Containers were misted daily during the initial establishment period (up to 14 days) and every 2 to 3 days thereafter until seedlings were harvested at 12 weeks old (Parkinson 2008).
In the production of plants for field experiments, stratified sulphur-flower buckwheat seeds were put in germination flats in a greenhouse and seedlings were transplanted twice to a final pot size of 4.5 in (11.4 cm). Containers were filled with 1:2:2 clay loam:perlite:peatmoss and watered for the first 7 weeks of growth. Plants were hardened for 5 weeks before outplanting on May 24 near Fort Collins, Colorado, where seedling survival and growth were good (Cox and Klett 1984).
Wildland Seeding And Planting
Sulphur-flower buckwheat is a mid- to late-seral, long-lived species recommended for pollinators (Walker and Shaw 2005; Eldredge et al. 2013). It grows well in full sun on well-drained soils in regions receiving 8 to 18 in (203–457 mm) annual precipitation (Walker and Shaw 2005; Young-Matthews 2012). It tolerates drought, saline and carbonate soil conditions and pH levels of 6.5 to 9 (Young-Matthews 2012; Eldredge et al. 2013). Plants are often competitive by the third post-seeding year (Stevens et al. 1996). Given the high variability of the species, it is important to match the habitat and variety of the plant material to the revegetation or restoration site (LBJWC 2019). See Seed Sourcing section.

Figure 15. Hydroseeding a native plant mix including sulphur-flower buckwheat at the Deschutes National Forest Ranger District Office in Bend, OR. The broadcast hyrdomulch and seed was applied in fall 2011 using sulphur-flower buckwheat and other native plant seed collected about 10 miles south. The site was irrigated. Photo: C. Powers, USFS.
Seeding. Sulphur-flower buckwheat can be seeded in the fall to break seed dormany when using local-adapted seed (Fig. 15). Seed can also be cold stratified (8-24 wks, depending on the elevation and climate of the collection site) and planted in early spring (Young-Matthews 2012). Drill seeding is recommended (Walker and Shaw 2005). With broadcast seeding, seeds should be covered by soil 0.25 in (2-5 mm) deep (Meyer 2008; Young-Matthews 2012). The recommended single-species broadcast seeding rate is 8 to 10 lbs/ac (9–11 kg/ha) (Young-Matthews 2012) and the species does well in seeding mixtures with other native species.
Monsen et al. (2004) reported fairly uniform sulphur-flower buckwheat germination over a 2-week period and good establishment when seeded as part of a mixture with other forbs, grasses, and shrubs (Fig. 16). Others indicated that germination was slow and recommended thick seeding in gardens or natural areas (LBJWC 2019). Sulphur-flower buckwheat established in seeding mixture experiments on a south-facing slope in Rocky Mountain National Park (Rowe and Brown 2008). Establishment was best in the first year in plots seeded with early (5 forbs, 1 grass) and late-seral (1 other forb, 2 shrubs, 2 grasses) species (0.5 seedling/1 × 1.5 m plot). Establishment in year two was best in plots seeded with only late-seral species (~0.8 seedling/plot). Sulphur-flower buckwheat seed was collected within 1.2 mi (2 km) of the experimental plots. Seeds were broadcast and raked in at a rate of 60 filled seeds/ft² (646/m²). The seed mix was evenly divided among the species in the mixture. Before seeding, all plots were treated with glyphosate, which reduced cheatgrass cover to 5% or less for the first year. In each year, sulphur-flower buckwheat seedlings were also present in the unseeded controls (~0.1 seedling/plot) (Rowe and Brown 2008).

Figure 16. An 8-year-old hydroseeded native plant mix including sulphur-flower buckwheat growing at the Deschutes National Forest Ranger District Office in Bend, OR. The site is a western juniper/antelope bitterbrush/bluebunch wheatgrass potential vegetation type. Photo: C. Powers, USFS.
Seeding restoration examples. Good establishment and survival of sulphur-flower buckwheat were reported in several restoration projects. Sulphur-flower buckwheat was one of the greatest biomass producing species in restoration treatments of pile burn scars in ponderosa pine-Douglas-fir. Burn scars were about 2 years old when treated to one of four pre-seeding treatments: hand-scarified (upper 4 in [10 cm] of soil tilled), wood chip mulch layer added (2–3 in [6–8 cm] deep), branch slash added to produce about 50% shade cover, or untreated. Sulphur-flower was absent from unseeded treated burn scars but when fall seeded at a rate of 10 PLS/ft² (108 PLS/m²), biomass of sulphur-flower buckwheat was 10 g/m² on scarified, 0.03 g/m² on wood chip, 1.7 g/m² on branches, and 4.7 g/m² on untreated burn scars (Shanklin 2014).
Sulphur-flower buckwheat seedling emergence and survival were high when seeded 0.4 in (1 cm) deep in spring-burned and unburned mountain big sagebrush with intermediate singleleaf pinyon cover (38%). Emergence and survival differences were not different between years, burned or unburned sites, or beneath trees, beneath shrubs, or in the vegetation interspaces. The site burned mid-May and because of high fuel moistures, consumption was patchy (Board et al. 2008).
Sulphur-flower buckwheat survival was greatest in weed-free conditions when seedings were evaluated in increasing weed levels on deer winter range along the South Fork of the Payette River in Boise County, Idaho (Table 11; Holmgren 1954). The site had coarse, loose, granite soils with low-moisture-retaining capacity. In November 1949 seed of sulphur-flower buckwheat and 26 other browse species were hand planted into furrows in a weed-free site (all weeds were controlled before seeding and each subsequent spring), broadleaf-weed site (cheatgrass removed but replaced by a stand of annual broadleaf weeds), and cheatgrass site (relatively undisturbed and cheatgrass dominated). Sulphur-flower buckwheat seed was not treated prior to planting and had high germination capacity. No insect or pathogen damage was noted (Holmgren 1954).
Table 11. Survival and height of sulphur-flower buckwheat seeded into sites with various degrees and types of weed pressure in Boise County, ID (Holmgren 1954).
|
Conditions |
Jun 1950 |
Sept 1950 |
May 1951 |
May 1952 |
May 1951 |
|
————-% Survival———— |
Average height (in) |
||||
|
Weed-free |
100 |
48 |
47 |
47 |
5 |
|
Broad-leaf weeds |
65 |
28 |
26 |
26 |
2 |
|
Cheatgrass |
16 |
10 |
5 |
5 |
2 |
At a very low precipitation desert shrub site in Colorado’s McInnis Canyon National Conservation Area, just one sulphur-flower buckwheat emerged within 2 years of being seeded. The site, which receives just 3 to 5 in (80–130 cm) of annual precipitation, was herbicide treated to reduce competition from annual grasses a year before seeding with a cone seeder on August 24. Emergence was limited for most seeded forbs (Grant-Hoffman et al. 2015).
Planting restoration examples. Several revegetation projects reported good sulphur-flower buckwheat survival and growth when transplants were used. For field experiments, researchers reported good survival and growth of sulphur-flower buckwheat plugs planted at a site near Boise, Idaho. Transplants were watered every 2 or 3 days in the summer of the first growing season (Parkinson and DeBolt 2005). When the density of neighboring cheatgrass plants reached 8/ft² (85/m²); however, the biomass of sulphur-flower buckwheat decreased by 90% or more, suggesting restoration success may improve with cheatgrass control (Parkinson 2008).
Greenhouse grown sulphur-flower buckwheat container stock was used successfully in the revegetation of roadside cuts in a mountain big sagebrush-antelope bitterbrush-cheatgrass community northwest of Reno, Nevada (Everett 1980). The roadside cut was terraced in summer 1975, hydroseeded with Siberian wheatgrass (Agropyron sibiricum) and crested wheatgrass (A. cristatum) the following fall, and planted with shrubs in February 1976 and 1977. Soils were neutral, non-salty, sandy loams with low organic matter (< 0.4%), organic nitrogen (5–11 lbs/ac [6–12 kg/ha]), and available phosphorus (< 5 ppm). Annual precipitation averaged about 8 in (210 mm). Sulphur-flower buckwheat was planted with 12 other species in 1976 and 1977 on north and south exposures. Twenty plants of each species were planted 7 ft (2 m) apart at each of 6 sites. When weeds or grasses were present, an 11 ft² (1 m²) area was scalped before planting. Paper cone-tainers were split along the seam before planting as a way to reduce frost heaving and provide soil contact. Sites were not watered at planting or any time after that. Survival was over 90% for sulphur-flower buckwheat in the first growing season and a little over 50% (range: about 20–85%) in the second. Sulphur-flower buckwheat produced its greatest cover on south slopes, and contributed most to overall coverage on north slopes where few species grew well. Three-year-old plants covered a total of 8.6 ft² (0.8 m²) horizontally and 6.9 ft² (0.6 m²) vertically on south slopes and 2.8 ft² (0.26 m²) horizontally and vertically on north slopes of the roadside cuts (Everett 1980).
Sulphur-flower buckwheat transplant survival and growth were good 3 years after transplanting on spoils from an open-pit sulfur mine on the eastern slope of the Sierra Nevada in Alpine County, California. Spoils were gravelly clays with low pH (4.1) and little available nitrogen, but free of toxic levels of metals. One-year-old stock (2 × 2 × 12 in [5 × 5 × 30 cm] paper tubes) of 11 shrub species and 1 tree were planted in October 1974 when plants had about 8 in² (50 cm²) crown cover. Transplants were placed at 6.5-ft (2 m) intervals in an 8-in (20 cm) deep furrow designed to catch precipitation. Plants were watered at the time of planting (1 gal [3.8 l]) and 2 days later with liquid fertilizer (NPK: 20-20-20, 1 gal). Shrub survival and growth were compared at sites with and without seeded grasses (native and nonnative species 11 lbs/ac [12 kg/ha]). By the third post-planting year, sulphur-flower buckwheat survival was 86% in unseeded and 73% in seeded plots. Although sulphur-flower buckwheat set seed by the third year, there were no seedling recruits. Grasses established the second year after seeding and were only found in close association with transplants. After 3 years, cover of individual sulphur-flower buckwheat plants was 39 in² (250 cm²) in the unseeded and 20 in² (130 cm²) in seeded plots (Everett et al. 1980).
Appendices
Appendix 1. Sulphur-flower buckwheat varieties and their elevation range, distribution, soil relationships, growth form, flower form and color, and flowering phenology (Reveal 2005).
|
Variety |
Elevation |
Range |
Soils/Plant Assoc |
Growth form |
Flower form, color |
Flowering phenology |
|
ahartii |
1,300–6,600 ft |
Paradise and Lumpkin Ridge areas of Butte Co, CA |
Serpentine soils |
Shrub
|
Compound umbel (3–4 branches), bright yellow |
June–Sept |
|
argus |
3,000–8,200 ft |
Siskiyou/Trinity Mtns in Josephine and Jackson Cos, OR; Del Norte, Glenn, Humboldt, Plumas, Siskiyou, and Trinity Cos, CA |
Serpentine soils |
Herb, spreading mat |
Umbel or compound umbel (2–4 branches), bright yellow |
June–Sept |
|
aureum |
4,600–11,200 ft |
CO, ID, MT, NV, OR, UT, WY |
Sandy to gravelly or rocky soils; mixed grasslands, sagebrush, PJ, and montane conifer |
Herb, compact mat |
Umbel, bright yellow |
June–Sept |
|
bahiiforme |
2,300–6,600 ft |
Widely scattered in Central Coast Ranges in Colusa, Contra Costa, Monterey, Napa, San Benito, Santa Clara, and Sonoma Cos and San Gabriel Mtns of Los Angeles County, CA |
Sandy to gravelly, mostly serpentine soils; oak woodlands and montane conifer |
Herb, spreading mat |
Compound umbel (2–5 branches), bright yellow |
July–Sept |
|
canifolium |
7,900–8,500 ft |
Infrequent in southern Sierra Nevada of Inyo and Tulare Cos and in Argus Mtns and Transverse Ranges of sw Kern and Los Angeles Cos, CA |
Sandy granitic soils; montane conifer |
Herb, spreading mat |
Umbel, bright yellow |
June–Sept |
|
chlorothamnus |
5,200–9,500 ft |
Scattered populations on eastern slope of Sierra Nevada in Inyo, s Mono, and ne Tulare Cos, CA |
Sandy granitic soils; sagebrush and montane conifer |
Subshrub/shrub 16–47 in |
Compound umbel (2–5 branches), bright yellow |
July–Sept |
|
cladophorum |
6,600–7,500 ft |
Rare; known only from Upper Geyser Basin, Old Faithful, and Madison Junction in Yellowstone National Park, WY and Teton Co, ID |
Sandy to gravelly soils; sagebrush and montane conifer |
Herb, compact mat |
Umbel, bright yellow |
June–Sept |
|
cognatum |
5,200–7,200 ft |
Infrequent in s-c Coconino, Gila, and Yavapai Cos, AZ. More frequent in greater Flagstaff area |
Sandy soils; sagebrush, oak woodlands, and montane conifer |
Herb, compact mat |
Compound umbel (2–4 branches), bright yellow |
July–Sept |
|
covillei |
9,800–11,800 ft |
Rare throughout its range along backbone of Sierra Nevada in Inyo and Tulare Cos, and White Mtns in Mono Co, CA |
Gravelly to rocky or talus slopes and ridges; high-elevation sagebrush, alpine conifer |
Herb, prostrate mat |
Compact umbel, bright yellow |
July–Sept |
|
desereticum |
5,000–10,800 ft |
S ID, sw MT, ne NV, n UT, sw WY; common only in UT and ID |
Sandy to gravelly slopes and ridges; mixed grasslands, sagebrush, oak woodlands, aspen, montane to subalpine conifer |
Herb, spreading mat |
Umbel, pale yellow, cream |
June–Sept |
|
devestivum |
2,600–5,900 ft |
Infrequent in Asotin and Columbia Cos, WA; Baker, Grant, and Union Cos, OR; Ada, Adams, Blaine, Lemhi, Valley, and Washington Cos, ID |
Sandy to gravelly flats and slopes; sagebrush, montane conifer |
Herb, spreading mat |
Compound umbel (2–4 branches), bright yellow |
June–Sept |
|
dichrocephalum |
3,900–11,200 ft |
Widespread from se OR, s ID, w WY, s to c CA, c NV, and n UT |
Sandy to gravelly soils; mixed grasslands, sagebrush, PJ, montane to subalpine conifer |
Herb, spreading mat |
Umbel, pale yellow to cream or white or greenish white |
June–Sept |
|
dumosum |
1,000–4,000 ft |
Widely scattered; Amador, Placer, Plumas, Shasta, and Siskiyou Cos, CA, and Jackson Co, OR |
Sandy to gravelly soils; mixed grasslands, oak woodlands, montane conifer |
Shrubs, round to erect |
Umbel, bright yellow |
June–Sept |
|
ellipticum |
2,300–7,900 ft |
Widely scattered but locally common in mtns of the Pacific Northwest (ID, MT, OR, WA) |
Sandy to gravelly soils; mixed grasslands, sagebrush, montane conifer |
Herb, compact mat |
Compound umbel (2–4 branches), bright yellow |
June–Sept |
|
furcosum |
4,000–9,900 ft |
Common in Sierra Nevada (Alpine, Amador, Calaveras, El Dorado, Fresno, Inyo, Kern, Madera, Mariposa, Mono, Nevada, Placer, Sierra, Tulare, and Tuolumne Cos, CA) and Mt. Rose/Slide Mtn area s Washoe Co NV |
Sandy to gravelly soils; sagebrush, oak woodlands, montane conifer |
Subshrub, spreading to rounded |
Compound umbel (2–4 branches), bright yellow |
June–Sept |
|
glaberrimum |
5,200–7,500 ft |
Localized, only known with certainty from Warner Mtns, Lake Co, OR, and Modoc Co, CA |
Sandy to gravelly soils; sagebrush, aspen, montane conifer |
Herb, compact mat |
Umbel, cream to white |
July–Sept |
|
goodmanii |
1,300–9,200 ft |
Common only in Waldo area, Josephine Co, OR but also in Benton, Deschutes, and Douglas Cos, OR, and Del Norte, Humboldt, and Siskiyou Cos, CA |
Sandy to gravelly serpentine soils; mixed grasslands, oak woodlands, montane conifer |
Herb, spreading to prostrate mat |
Umbel, bright yellow |
May–Sept |
|
haussknechtii |
3,300–10,200 ft |
High-elevation peaks in nc OR (Benton, Clackamas, Hood River, and Wasco Cos) and sc WA (Kittitas and Yakima Cos); common on Mt. Hood and Mt. Adams |
Volcanic, sandy to gravelly slopes and ridges; mixed grasslands, sagebrush, montane to subalpine conifer |
Herb, prostrate, sprawling mat |
Compact, umbel, bright yellow |
June–Sept |
|
humistratum |
5,600–9,200 ft |
Restricted to exposed serpentine sites (White Mtn, Eddy-Scott Mtn, Marble Mtns, and Mt. Shasta) in Siskiyou and Trinity Cos, CA |
Gravelly serpentine slopes and ridges; montane conifer woodlands |
Herb, prostrate mat |
Umbel, bright yellow |
June–Sept |
|
hypoleium |
3,000–6,900 ft |
Restricted to Chelan and Kittitas Cos, WA but extends from Mt. Stuart Range south to Bald Mountain area west of Ellensburg, WA |
Gravelly to rocky slopes and ridges; high-elevation sagebrush, montane to subalpine conifer |
Herb, compact mat |
Umbel, bright yellow |
June–Sept |
|
juniporinum |
4,300–8,200 ft |
Widely scattered and disjunct populations in isolated desert mtn ranges from s UT, nc AZ, s NV, and CA |
Sandy to gravelly soils; saltbush, sagebrush, PJ, and occasionally montane conifer |
Subshrub or shrub, spreading to erect |
Compound umbel (2–5 branches), cream, whitish, pale yellow, greenish yellow |
June-Oct |
|
lautum |
2,600–3,000 ft |
Restricted to Scott Valley of Siskiyou Co, CA |
Sandy to gravelly flats; oak and conifer woodlands |
Herb, spreading mat |
Compound umbel, bright yellow |
July–Sept |
|
majus |
2,600–11,500 ft |
Widespread and common in the Rocky Mtns (Alta, BC; CO, ID, MT, UT, WA, WY) |
Sandy to gravelly soils; mixed grasslands, sagebrush, oak woodlands, aspen, montane to subalpine conifer, mountain meadows, alpine tundra |
Herb, compact mat 4–20 × 8–40 in |
Umbel, cream |
June–Sept |
|
minus |
5,900–10,200 ft |
Rare; San Bernardino and San Gabriel Mtns in Los Angeles and San Bernardino Cos, CA |
Gravelly to rocky or talus slopes and ridges; sagebrush, montane to subalpine conifer |
Herb, dense, prostrate mat |
Compact umbel, lemon yellow, yellow-red to rose-red |
July–Sept |
|
modocense |
700–8,200 ft |
Common e of the Cascade Range in c OR and n CA; less frequent in n NV and sw ID |
Sandy to gravelly soils; mixed grasslands, sagebrush, PJ, montane conifer |
Herb, mat |
Umbel, bright yellow |
June–Sept |
|
mohavense |
3,900–5,200 ft |
Known only from Black Rock and Wolf Hole Mtns in Mohave Co, AZ |
Sandy to gravelly soils; sagebrush, oak woodlands, PJ, montane conifer |
Herb, spreading mat |
Umbel, bright yellow |
May–June |
|
munzii |
4,900–9,500 ft |
Mainly in Transverse Ranges from Santa Barbara, Ventura, Kern, San Bernardino, Los Angeles Cos, CA; disjunct population in San Jacinto Mtns, Riverside Co and Laguna Mtns, San Diego Co, CA |
Sandy to gravelly soils; sagebrush, oak woodlands, montane conifer |
Herb, spreading mat |
Compound umbel (2–4 branches), bright yellow |
June–Sept |
|
nevadense |
3,300–11,200 ft |
Widespread and common in CA, NV, and se OR |
Sandy to gravelly soils; mixed grasslands, sagebrush, PJ, montane to subalpine conifer |
Subshrub, mostly spreading |
Umbel, bright yellow |
June–Sept |
|
polyanthum |
2,600–4,900 ft |
CA |
Serpentine soils, oak woodlands, montane conifer |
Shrub, round and open |
Compound umbel (1–3 branches), bright yellow |
June–Sept |
|
porter |
7,900–12,100 ft |
Elko, Lander, and Nye Cos, NV; Uinta and Wasatch Mtns in UT; Rocky Mtns in CO |
Rocky slopes and ridges; high-elevation sagebrush, meadows, subalpine to alpine conifer |
Herb, cespitose mat |
Umbel, bright yellow |
July–Sept |
|
ramulosum |
5,200–8,900 ft |
Rare; mainly along Rocky Mtn Front Range and in Jefferson and Larimer Cos, CO |
Sandy to gravelly slopes; sagebrush, montane conifer |
Herb, compact mat |
Compound umbel (1–3 branches), bright yellow |
June–Sept |
|
sandbergii |
1,000–3,900 ft |
Scattered in foothills and low mtns of Cascade Ranges in nc OR (Hood River and Wasco Cos) and c WA (Chelan, Kittitas, Okanogan, and Yakima Cos) |
Sandy to gravelly slopes and ravines; sagebrush, montane conifer |
Subshrub, sprawling |
Umbel, bright yellow |
May–Aug |
|
smallianum |
2,300–6,600 ft |
North Coast Ranges in Glenn, Lake, Mendocino, Sonoma, Tehama, and Trinity Cos, CA |
Sandy to gravelly, mostly serpentine flats and slopes; oak and montane conifer woodlands |
Herb, spreading mat |
Compound umbel (1–3 branches), bright yellow |
July–Sept |
|
speciosum |
300–2,600 ft |
Few scattered locations in Humboldt and Trinity Cos, CA |
Serpentine flats and slopes; oak woodlands and montane conifer |
Shrub, spreading to rounded |
Compound umbel (2–4 branches), bright yellow |
June–Sept |
|
stragulum |
4,600–8,200 ft |
Common in s ID foothills and mtns in Snake River Plains; Teton Co, WY; Elko Co, NV |
Sandy to gravelly or rocky soils; mixed grasslands, sagebrush, PJ, and montane conifer |
Herb, spreading mat |
Umbel, bright yellow |
May–Sept |
|
subaridum |
3,900–10,200 ft |
Widespread and often common throughout an extensive range of se CA, s NV, n AZ, UT, and sw CO |
Sandy to gravelly soils; mixed grasslands, saltbush, sagebrush, PJ, oak woodlands, montane conifer |
Subshrub or shrub, spreading to erect |
Compound umbel (2–5 branches), bright yellow |
June–Oct |
|
torreyanum |
5,900–7,900 ft |
Only in NV (Sierra Nevada in Placer and Sierra Cos) |
Sandy to gravelly granitic slopes; buckbrush, manzanita, montane conifer |
Herb, compact mat |
Umbel, bright yellow |
July–Sept |
|
umbellatum |
3,300–10,200 ft |
Widespread and common in CO, ID, MT, UT, and WY |
Sandy to gravelly flats and slopes; mixed grasslands, sagebrush, scrub oak and montane conifer |
Herb, spreading mat |
Umbel, bright yellow |
June–Sept |
|
vernum |
4,600-7,200 ft 1,400-2,200 m |
Scattered populations in northern Nye Co, NV |
Sandy to gravelly, often volcanic soils; saltbush, sagebrush |
Shrub, dome-shaped |
Umbel, pale to bright yellow |
May–July |
|
versicolor |
6,200–10,800 ft |
Infrequent and scattered in s NV Mtns (Clark, Eureka, Lincoln, Lyon, Nye, and White Pine Cos) and Inyo and Mono Cos, CA |
Gravelly to rocky flats, slopes, and ridges; sagebrush, montane conifer |
Herb, spreading to prostrate mat |
Umbel or compound umbel, yellow, becoming brown to rose or pink |
June–Sept |
Appendix 2. Sulphur-flower buckwheat varieties and their synonyms. Nomenclature follows Reveal (2005).
|
Variety |
Synonyms |
|
aureum |
E. glaberrimum Gandoger var. aureum Gandoger; E. neglectum Greene; E. umbellatum var. glabratum S. Stokes; E. umbellatum var. intectum A. Nelson; E. umbelliferum Small |
|
bahiiforme |
E. polyanthum Bentham var. bahiiforme Torrey & A. Gray; E. u. subsp. bahiaeforme (Torr. & A. Gray) Munz |
|
cladophorum |
E. rydbergii Greene |
|
cognatum |
E. cognatum Greene; E. u. subsp. cognatum (Greene) S. Stokes |
|
covillei |
E. covillei Small; E. u. subsp. covillei (Small) Munz |
|
dichrocephalum |
E. latum Small ex Rydberg; E. u. subsp. aridum (Greene) S. Stokes; E. u. var. aridum (Greene) C. L. Hitchcock |
|
dumosum |
E. dumosum Greene; E. u. subsp. dumosum (Greene) S. Stokes |
|
ellipticum |
E. ellipticum Nuttall; E. croceum Small; E. stellatum Bentham; E. u. var. chrysanthum Gandoger; E. u. var. croceum (Small ex Rydberg) S. Stokes; E. u. subsp. stellatum (Bentham) S. Stokes; E. u. var. stellatum (Bentham) M. E. Jones |
|
glaberrimum |
E. glaberrimum Gandoger |
|
haussknechtii |
E. haussknechtii Dammer; E. u. subsp. haussknechtii (Dammer) S. Stokes |
|
hypoleium |
E. u. subsp. hypoleium |
|
majus |
E. subalpinum Greene; E. u. subsp. majus (Hooker) Piper; E. u. subsp. subalpinum (Greene) S. Stokes |
|
minus |
E. u. subsp. minus (I. M. Johnston) Munz |
|
modocense |
E. modocense |
|
munzii |
E. u. subsp. munzii (Reveal) Thorne ex Munz |
|
polyanthum |
E. polyanthum Bentham; E. u. subsp. polyanthum (Bentham) S. Stokes |
|
porter |
E. porteri Small |
|
smallianum |
E. smallianum A. Heller |
|
speciosum |
E. speciosum Drew |
|
subaridum |
E. biumbellatum Rydberg; E. u. subsp. ferrissii (A. Nelson) S. Stokes; E. u. subsp. subaridum (S. Stokes) Munz |
|
torreyanum |
E. torreyanum A. Gray |
Acknowledgements
Funding for Western Forbs: Biology, Ecology, and Use in Restoration was provided by the USDI BLM Great Basin Native Plant Materials Ecoregional Program through the Great Basin Fire Science Exchange. Great thanks to the chapter reviewers: Robert L. Johnson, Brigham Young University; Sarah Kulpa, US Fish and Wildlife Service; Sarah Barga, US Forest Service.
This research was supported in part by the USDA Forest Service, Rocky Mountain Research Station. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA or U.S. Government determination or policy.
Literature Cited
Abella, S.R.; Cayenne Engel, E.; Springer, J.D.; Wallace Covington, W. 2012. Relationships of exotic plant communities with native vegetation, environmental factors, disturbance, and landscape ecosystems of Pinus ponderosa forests, USA. Forest Ecology and Management. 271: 65-74.
Anjozian, L.N. 2008. Searching, witnessing, testing: Plants and fire in southern California. Joint Fire Science Program Fire Science Brief. Project: 01B-3-3-28. Boise, ID: Joint Fire Science Program. 6 p. Available: http://www.firescience.gov/projects/briefs/01B-3-3-28_FSBrief20.pdf
Archibald, C. 2006. Seed production protocols for Anaphalis margaritacea, Eriophyllum lanatum, and Eriogonum umbellatum. Native Plants Journal. 7(1): 47-51.
Association of Official Seed Analysts [AOSA]. 2010. AOSA/SCST Tetrazolium testing handbook. Contribution No. 29. Lincoln, NE: Association of Official Seed Analysts.
Auckland, J.N.; Debinski, D.M.; Clark, W.R. 2004. Survival, movement, and resource use of the butterfly Parnassius clodius. Ecological Entomology. 29(2): 139-149.
Austin, D.D.; Urness, P.J. 1986. Effects of cattle grazing on mule deer diet and area selection. Journal of Range Management. 39(1): 18-21.
Ayer, W.A.; Browne, L.M.; Kasitu, G.C. 1990. Metabolites of Eriogonum umbellatum. Planta Medica. 56(3): 336-336.
Barner, J. 2009a. Propagation protocol for production of propagules (seeds, cuttings, poles, etc.) Eriogonum umbellatum Torr. seeds. Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://npn.rngr.net/propagation/protocols [Accessed 2019 April 4].
Bartmann, R.M. 1983. Composition and quality of mule deer diets on pinyon-juniper winter range, Colorado. Journal of Range Management. 36(4): 534-541.
Basey, A.C.; Fant, J.B.; Kramer, A.T. 2015. Producing native plant materials for restoration: 10 rules to collect and maintain genetic diversity. Native Plants Journal. 16(1): 37-53.
Beatley, J.C. 1976. Vascular plants of the Nevada Test Site and central-southern Nevada: Ecologic and geographic distributions. Springfield, VA: U.S. Department of Commerce, Office of Technical Information, Technical Information Center. 308 p.
Blanke, T.; Woodruff, H. 2011. Propagation protocol for production of propagules (seeds, cuttings, poles, etc.) Eriogonum umbellatum plants. Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://npn.rngr.net/propagation/protocols [Accessed 2019 April 4].
Board, D.I.; Chambers, J.C.; Wright, J.G. 2008. Effects of spring prescribed fire in expanding pinyon-juniper woodlands on seedling establishment of sagebrush species. Natural Resources and Environmental Issues. 16(20): 1-10.
Bower, A.D.; St. Clair, J.B.; Erickson, V. 2014. Generalized provisional seed zones for native plants. Ecological Applications. 24(5): 913-919.
Bunnell, K.D.; Flinders, J.T.; Mitchell, D.L.; Warder, J.H. 2004. Occupied and unoccupied sage grouse habitat in Strawberry Valley, Utah. Journal of Range Management. 57(5): 524-531.
Bunting, S.C.; Kingery, J.L.; Strand, E. 1999. Effects of succession on species richness of the western juniper woodland/sagebrush steppe mosaic. In: Monsen, S.B.; Stevens, R., comps. Proceedings: Ecology and management of pinyon-juniper communities within the Interior West: Sustaining and restoring a diverse ecosystem. 1997 September 15-18; Provo, UT. Proc. RMRS-P-9. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 76-81.
Burke, I.C.; Reiners, W.A.; Olson, R.K. 1989. Topographic control of vegetation in a mountain big sagebrush steppe. Vegetatio. 84(2): 77-86.
Burkle, L.; Irwin, R. 2009. The importance of interannual variation and bottom-up nitrogen enrichment for plant-pollinator networks. Oikos. 118(12): 1816-1829.
Chirco, E.; Turner, T. 1986. Species without AOSA testing procedures. The Newsletter of the Association of Official Seed Analysts. 60(2): 2-66. Updated April 2007.
Clary, W.P. 1989. Test of RPA production coefficients and local assumptions for the pinyon-juniper ecosystem in central Utah. Res. Pap. INT-403. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 11 p.
Cole, D.N. 1988. Disturbance and recovery of trampled montane grassland and forests in Montana. Res. Pap. INT-389. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station. 37 p.
Consortium of Pacific Northwest Herbaria [CPNWH]. 2017. Seattle, WA: University of Washington Herbarium, Burke Museum of Natural History and Culture. http://www.pnwherbaria.org/index.php
Coville, F.V. 1897. Notes on the plants used by the Klamath Indians of Oregon. U.S. National Herbarium. 5(2): 87-108.
Cox, R. 2016. Smoke-induced germination of Great Basin native forbs. In: Kilkenny, F; Edwards, F.; Malcomb, A., eds. Great Basin Native Plant Project: 2015 Progress Report. Boise, ID: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 78-82.
Cox, R.A.; Klett, J.E. 1984. Evaluation of some indigenous western plants for xeric landscapes. HortScience. 19(6): 856-858.
Craighead, J.J.; Craighead, F.C. Jr.; Davis, R.J. 1963. A field guide to Rocky Mountain wildflowers from northern Arizona and New Mexico to British Columbia. Boston, MA: Houghton Mifflin Company. 277 p.
Davis, A.S.; Fisk, M.R.; Apostol, K.G.; Pete, K.W. 2014. Dynamics of germination and cold hardiness for Great Basin plants. In: Kilkenny, F.; Shaw, N.; Gucker, C., eds. Great Basin Native Plant Project: 2013 Progress Report. Boise, ID: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 39-46.
Day, T.; Wright, R.G. 1985. The vegetation types of Craters of the Moon National Monument. Bull. 38. Moscow, ID: University of Idaho, College of Forestry, Wildlife, and Range Sciences, Forest, Wildlife and Range Experiment Station. 6 p.
Day, T.A.; Wright, R.G. 1989. Positive plant spatial association with Eriogonum ovalifolium in primary succession on cinder cones: Seed-trapping nurse plants. Vegetatio. 80(1): 37-45.
Dayton, W.A. 1960. Notes on western range forbs: Equisetaeae through Fumariaceae. Washington, DC: U.S. Department of Agriculture, Forest Service. 254 p.
Debinski, D.M.; Kindscher, K. 1994. A remote sensing and GIS based model of habitat as a predictor of biodiversity. University of Wyoming National Park Service Research Center Annual Report. 18(2): 3-16.
Debinski, D.M.; Szcodronski, K.; Germino, M. 2014. Simulated expected changes in pollinator resources as a function of climate change. University of Wyoming National Park Research Center Annual Report. 37(5): 29-33.
Dunn, P.O.; Braun, C.E. 1986. Summer habitat use by adult female and juvenile sage grouse. The Journal of Wildlife Management. 50(2): 228-235.
Dvorak, B.; Volder, A. 2010. Green roof vegetation for North American ecoregions: A literature review. Landscape and Urban Planning. 96(4): 197-213.
Dyer, D. 2005. Plant guide: Sulphur-flower buckwheat (Eriogonum umbellatum Torr.). Lockeford, CA: U.S. Department of Agriculture, Natural Resources Conservation Service, Lockeford Plant Materials Center. 3 p.
Eldredge, E.; Novak-Echenique, P.; Heater, T.; Mulder, A.; Jasmine, J. 2013. Plants for pollinator habitat in Nevada. Tech. Note NV 57. Reno, NV: U.S. Department of Agriculture, Natural Resources Conservation Service. 65 p.
European Native Seed Conservation Network [ENSCONET]. 2009. ENSCONET seed collecting manual for wild species. Edition 1: 32 p.
Everett, P. 1957. A summary of the culture of California plants at the Rancho Santa Ana Botanic Garden 1927-1950. Claremont, CA: The Rancho Santa Ana Botanic Garden. 223 p.
Everett, R.L. 1980. Use of containerized shrubs for revegetating arid roadcuts. Reclamation Review. 3(1): 33-40.
Everett, R.L.; Meeuwig, R.O.; Butterfield, R.I. 1980. Revegetation of untreated acid spoils: Leviathan mine, Alpine County, California. California Geology. 32(1): 8-10.
Farr, D.F.; Rossman, A.Y. 2017. Fungal databases, U.S. National Fungus Collections. U.S. Department of Agriculture, Agricultural Research Service. https://nt.ars-grin.gov/fungaldatabases/.
Ferris, C.D. 1973. Life history of Callophrys sheridanii sheridanii Lycaenidae and notes on other species. Journal of the Lepidopteristis’ Society. 27(4): 279-283.
Fisk, M.R.; Apostol, K.G.; Ross-Davis, A.L.; Cahoy, D.O.; Davis, A.S. 2019. Informing native plant sourcing for ecological restoration: Cold-hardiness dynamics, flowering phenology, and survival of Eriogonum umbellatum. Restoration Ecology. 27(3): 616-625.
Fraas, W.W. 1992. Bitterbrush growth and reproductive characters in relation to browsing in southwest Montana. Bozeman, MT: Montana State University. Thesis. 137 p.
Franklin, J.F.; Dyrness, C.T. 1973. Natural vegetation of Oregon and Washington. Gen. Tech. Rep. PNW-8. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 452 p.
Grant-Hoffman, N.; Parr, S.; Blanke, T. 2015. Native plant species field evaluation in salt desert: Good materials, bad situation. Native Plants Journal. 16(2): 87-95.
Hanson, H.C.; Dahl, E. 1957. Some grassland communities in the mountain-front zone in northern Colorado. Vegetatio. 7(4): 249-270.
Hart, J.A. 1981. The ethnobotany of the northern Cheyenne Indians of Montana. Journal of Ethnopharmacology. 4(1): 1-55.
Hazlett, D.L.; Sawyer, N.W. 1998. Distribution of alkaloid-rich plant species in shortgrass steppe vegetation. Conservation Biology. 12(6): 1260-1268.
Hickman, J.C., ed. 1993. The Jepson manual: Higher plants of California. Berkeley, CA: University of California Press. 1400 p.
Hitchcock, C.L.; Cronquist, A. 2018. Flora of the Pacific Northwest: An illustrated manual. Second Ed. Giblin, D.E.; Legler, B.S.; Zika, P.F.; Olmstead, R.G., eds. Seattle, WA: University of Washington Press. 882 p.
Hobbs, N.T.; Baker, D.L.; Ellis, J.E.; Swift, D.M. 1981. Composition and quality of elk winter diets in Colorado. Journal of Wildlife Management. 45(1): 156-171.
Holch, A.E.; Hertel, E.W.; Oakes, W.O.; Whitwell, H.H. 1941. Root habits of certain plants of the foothill and alpine belts of Rocky Mountain National Park. Ecological Monographs. 11(3): 327-345.
Holmgren, R.C. 1954. A comparison of browse species for revegetation of big-game winter ranges in southwestern Idaho. Res. Pap. No. 3. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 12 p.
Horner, M.A. 2001. Vascular flora of the Glass Mountain region, Mono County, California. Aliso. 20(2): 75-105.
Howe, G.; St, Clair, B.; Bachelet, D. 2017. Seedlot Selection Tool. Corvallis, OR: Conservation Biology Institute. https://seedlotselectiontool.org/sst/
Jackson, L.E. 1985. Floristic analysis of the distribution of ephemeral plants in treeline areas of the western United States. Arctic and Alpine Research. 17(3): 251-260.
James, D.G.; Nunnallee, D. 2011. Life histories of Cascadia butterflies. Corvallis, OR: Oregon State University Press. 447 p.
James, D.G.; Seymour, L.; James, T.S. 2014. Population biology and behavior of the imperiled Philotilla leona (Lycaenidae) in south central Oregon. Journal of the Lepidopterists Society. 68(4): 264-273.
James, D.G.; Seymour, L.; Lauby, G.; Buckley, K. 2014. Beneficial insects attracted to native flowering buckwheats (Eriogonum Michx) in central Washington. Environmental Entomology. 43(4): 942-948.
Johnson, B.K.; Schultz, R.D.; Bailey, J.A. 1978. Summer forages of mountain goats in the Sawatch Range, Colorado. Journal of Wildlife Management. 42(3): 636-639.
Johnson, C.G.; Swanson, D.K. 2005. Bunchgrass plant communities of the Blue and Ochoco Mountains: A guide for managers. Gen. Tech. Rep. PNW-GTR-641. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 119 p.
Johnson, R.C.; Bradley, V.; Cashman, M. 2018. Conservation, adaptation, and seed zones for key Great Basin species. In: Kilkenny, F; Edwards, F.; Irwin, J.; Barga, S., eds. Great Basin Native Plant Project: 2017 Progress Report. Boise, ID: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 38-43.
Johnson, R.C.; Bradley, V.; Cashman, M.; Love, S. 2017. Conservation, adaptation, and seed zones for key Great Basin species. In: Kilkenny, F; Edwards, F.; Irwin, J.; Barga, S., eds. Great Basin Native Plant Project: 2016 Progress Report. Boise, ID: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 41-46.
Kay, B.L.; Graves, W.L.; Young, J.A. 1988. Long-term storage of desert shrub seed. Mojave Revegetation Notes No 23. Davis, CA: University of California, Agronomy and Range Science. 22 p.
Klott, J.H.; Lindzey, F.G. 1990. Brood habitats of sympatric sage grouse and Columbian sharp-tailed grouse in Wyoming. Journal of Wildlife Management. 54(1): 84-88.
Koniak, S. 1985. Succession in pinyon-juniper woodlands following wildfire in the Great Basin. Great Basin Naturalist. 45(3): 556-566.
Kooiman, M.; Linhart, Y.B. 1986. Structure and change in herbaceous communities of four ecosystems in the Front Range, Colorado, U.S.A. Arctic and Alpine Research. 18(1): 97-110.
Kramer, A.T.; Foxx, A. 2016. Propagation protocol for production of propagules (seeds, cuttings, poles, etc.) Eriogonum umbellatum seeds. Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://npn.rngr.net/propagation/protocols [Accessed 2019 April 4].
Kufeld, R.C.; Wallmo, O.C.; Feddema, C. 1973. Foods of the Rocky Mountain mule deer. Res. Pap. RM-111. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 31 p.
Lady Bird Johnson Wildflower Center [LBJWC]. 2019. Eriogonum umbellatum Torr. Native Plant Database. Austin, TX: Lady Bird Johnson Wildflower Center. https://www.wildflower.org/plants-main [Accessed 2019 April 10].
Lambert, S. 2005. Guidebook to the seeds of native and non-native grasses, forbs and shrubs of the Great Basin. Boise, ID: U.S. Department of the Interior, Bureau of Land Management, Idaho State Office. 136 p.
Love, B.; Cane, J. 2019. Mortality and flowering of Great Basin perennial forbs after experimental burning: Implications for wild bees. Rangeland Ecology and Management. 72(2): 310-317.
Luna, T.; Corey, S. 2008. Propagation protocol for production of container (plug) Eriogonum umbellatum Torr. plants 172 ml conetainers. Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://npn.rngr.net/propagation/protocols [Accessed 2019 April 4].
Majerus, M. 1991. Yellowstone National Park-Bridger Plant Materials Center native plant program. In: Rangeland Technology and Equipment Council, ed. 1991 Annual Report. 9222-2808-MTDC. Missoula, MT: U.S. Department of Agriculture, Forest Service, Missoula Technology and Development Center, Technology and Development Program: 17-22.
Marchand, D.E. 1973. Edaphic control of plant distribution in the White Mountains, eastern California. Ecology. 54(2): 233-250.
Martin, A.C.; Zim, H.S.; Nelson, A.L. 1951. American wildlife and plants: A guide to wildlife food habits. New York, NY: Dover Publications. 500 p.
McGee, J.M. 1976. Some effects of fire suppression and prescribed burning on birds and small mammals in sagebrush. Jackson Hole Research Station Annual Report. 10: 31-32.
Meyer, S.E. 2008. Eriogonum Michx.; wild-buckwheat, buckwheatbrush. In: Bonner, F.T.; Karrfalt, R.P., eds. The woody plant seed manual. Agriculture Handbook 727. Washington, DC: U.S. Department of Agriculture, Forest Service: 499-503.
Meyer, S.E.; Kjelgren, R.K.; Morrison, D.G.; Varga, W.A. 2009. Chapter 7: The plant palette. In: Landscaping on the new frontier: Waterwise design for the Intermountain West. Logan, UT: Utah State University Press: 159-226.
Meyer, S.E.; Paulsen, A. 2000. Chilling requirements for seed germination of 10 Utah species of perennial wild buckwheat (Eriogonum Michx. [Polygonaceae]). Native Plants Journal. 1(1): 18-24.
Millar, C.I.; Westfall, R.D.; Delany, D.L. 2013. New records of marginal locations for American pika (Ochotona princeps) in the western Great Basin. Western North American Naturalist. 73(4): 457-476.
Miller, R.F.; Chambers, J.C.; Pyke, D.A.; Pierson, F.B.; Williams, C.J. 2013. A review of fire effects on vegetation and soils in the Great Basin Region: Response and ecological site characteristics. Gen. Tech. Rep. RMRS-GTR-308. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 126 p.
Mills, H.B. 1937. A preliminary study of the bighorn of Yellowstone National Park. Journal of Mammalogy. 18(2): 205-212.
Moerman, D. 2003. Native American ethnobotany: A database of foods, drugs, dyes, and fibers of Native American peoples, derived from plants. Dearborn, MI: University of Michigan. http://naeb.brit.org
Monsen, S.B.; Stevens, R.; Shaw, N.L. 2004. Chapter 23. Shrubs of other families. In: Monsen, S.B.; Stevens, R.; Shaw, N.L., comps. Restoring western ranges and wildlands, vol. 2. Gen. Tech. Rep. RMRS-GTR-136-vol-2. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 597-698.
Mueggler, W.F. 1983. Variation in production and seasonal development of mountain grasslands in western Montana. Res. Pap. INT-316. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 16 p.
Neely, E.E.; Barkworth, M.E. 1984. Vegetation on soils derived from dolomite and quartzite in the Bear River Range, Utah: A comparative study. Bulletin of the Torrey Botanical Club. 111(2): 179-192.
Nelson, G.H.; Westcott, R.L. 1976. Notes on the distribution, synonymy, and biology of Buprestidae (Coleoptera) of North America. The Coleopterists Bulletin. 30(3): 273-284.
O’Brien, M.H. 1980. The pollination biology of a pavement plain: Pollinator visitation patterns. Oecologia. 47(2): 213-218.
Ogle, D.; Cane, J.; Fink, F.; St. John, L.; Stannard, M.; Dring, T. 2007. Plants for pollinators in the Intermountain West. Plant Materials Tech Note 2. Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 22 p.
Ogle, D.; St. John, L.; Stannard, M.; Holzworth, L. 2012. Conservation plant species for the Intermountain West. Plant Materials Technical Note 24. Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 57 p.
Ogle, D.; Tilley, D.; Cane, J.; St. John, L.; Fullen, K.; Stannard, M.; Pavek, P. 2011. Plants for pollinators in the Intermountain West. Plant Materials Tech. Note 2A. Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 40 p.
Oldemeyer, J.L.; Martin, S.J.; Woodis, S.G. 1983. A preliminary report on the effects of a deferred-rotation grazing system on wildlife at the Sheldon National Wildlife Refuge. Cal-Neva Wildlife Transactions 1983: 26-42.
Omernik, J.M. 1987. Ecoregions of the conterminous United States. Map (scale 1:7,500,000). Annals of the Association of American Geographers. 77(1): 118-125.
Parkinson, H. 2003. Landscaping with native plants of the Intermountain Region. Tech. Ref. 1730-3. Boise, ID: U.S. Department of the Interior, Bureau of Land Management. 46 p.
Parkinson, H.; DeBolt, A. 2005. Propagation protocol for production of container (plug) Eriogonum umbellatum Torr. plants. Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://npn.rngr.net/propagation/protocols [Accessed 2019 April 4].
Parkinson, H.; Zabinski, C. 2009. Growth characteristics of five Great Basin forbs and interactions between forbs and grasses. In: Shaw, N.L.; Pellant, M., eds. Great Basin Native Plant Project: 2008 Progress Report. Boise, ID: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 96-105.
Parkinson, H.; Zabinski, C.; Shaw, N. 2013. Impact of native grasses and cheatgrass (Bromus tectorum) on Great Basin forb seedling growth. Rangeland Ecology and Management. 66(2): 174-180.
Parkinson, H.A. 2008. Impacts of native grasses and cheatgrass on Great Basin forb development. Bozeman, MT: Montana State University. Thesis. 73 p.
Parris, C.; Shock, C.C.; Feibert, E.; Shaw, N. 2010. Sulphur-flower buckwheat (Eriogonum umbellatum) ERUM. EM 9017. Corvallis, OR: Oregon State University Extension Service. 4 p.
Pate, V.S.L. 1937. Studies in the pemphredonine Wasps (Hymenoptera: Sphecidae). I. New genera and species of the Ammoplanoid complex. Transactions of the American Entomological Society. 63(2): 89-125.
Patten, D.T. 1963. Vegetational pattern in relation to environments in the Madison Range, Montana. Ecological Monographs. 33(4): 375-406.
Pavek, P.; Erhardt, B.; Heekin, T.; Old, R. 2012. Forb seedling identification guide for the Inland Northwest: Native, introduced, invasive, and noxious species. Pullman, WA: U.S. Department of Agriculture, Natural Resources Conservation Service, Pullman Plant Materials Center. 144 p.
Peterson, K.M.; Billings, W.D. 1982. Growth of alpine plants under controlled drought. Arctic and Alpine Research. 14(3): 189-194.
Plant Conservation Alliance [PCA]. 2015. National seed strategy for rehabilitation and restoration 2015-2020. Washington, DC: U.S. Department of the Interior, Bureau of Land Management. 52 p.
Powell, D.C.; Johnson, C.G., Jr.; Crowe, E.A.; Wells, A.; Swanson, D.K. 2007. Potential vegetation hierarchy for the Blue Mountains section of northeastern Oregon, southeastern Washington, and west-central Idaho. Gen. Tech. Rep. PNW-GTR-709. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 87 p.
Pratt, G.F.; Ballmer, G.R. 1991. Three biotypes of Apodemia mormo (Riodinidae) in the Mojave Desert. Journal of the Lepidopterists Society. 45(1): 46-57.
Ralphs, M.H.; Pfister, J.A. 1992. Cattle diets in tall forb communities on mountain rangelands. Journal of Range Management. 45(6): 534-537.
Ramaley, F. 1916. Dry grassland of a high mountain park in northern Colorado. The Plant World. 19(9): 249-270.
Rau, B.M.; Blank, R.R.; Chambers, J.C.; Johnson, D.W. 2007. Prescribed fire in a Great Basin sagebrush ecosystem: Dynamics of soil extractable nitrogen and phosphorus. Journal of Arid Environments. 71(4): 362-375.
Rau, B.M.; Chambers, J.C.; Blank, R.R.; Johnson, D.W. 2008. Prescribed fire, soil, and plants: Burn effects and interactions in the central Great Basin. Rangeland Ecology and Management. 61(2): 169-181.
Reveal, J.L. 2003. Eriogonum as a rock garden plant. Available: http://www.plantsystematics.org/reveal/pbio/eriog/eriogarden.html [Accessed 2019 May 1].
Reveal, J.L. 2005. Eriogonum Michaux. In: Flora of North America Editorial Committee, ed. Flora of North America North of Mexico. Volume 5 Magnoliophyta: Caryophyllidae, part 2. Flora of North America. http://www.efloras.org
Reveal, J.L. 2014. Eriogonum manual for the Eriogonum Society. Unpublished manual distributed at The Eriogonum Workshop. 2014 June 22-24. Twin Falls, ID. Manual on file at Boise, ID: U.S. Department of Agriculture, Forest Service, Boise Aquatic Sciences Laboratory: 526-618.
Rose, R.; Chachulski, C.E.C.; Haase, D.L. 1998. Propagation of Pacific Northwest native plants. Corvallis, OR: Oregon State University Press. 248 p.
Rowe, H.I.; Brown, C.S. 2008. Native plant growth and seedling establishment in soils influenced by Bromus tectorum. Rangeland Ecology and Management. 61(6): 630-639.
Royal Botanic Gardens Kew [RBG Kew]. 2017. Seed Information Database (SID). Version 7.1. http://data.kew.org/sid/
Sabinske, D.W.; Knight, D.H. 1978. Variation within the sagebrush vegetation of Grand Teton National Park, Wyoming. Northwest Science. 52(3): 195-204.
Safford, H.D.; Viers, J.H.; Harrison, S.P. 2005. Serpentine endemism in the California flora: A database of serpentine affinity. Madrono. 52(4): 222-257.
Sampangi, R.K.; Mohan, S.K.; Shock, C.C. 2012. Etiology, epidemiology and management of diseases of native wildflower seed production. In: Shaw, N.L.; Pellant, M., eds. Great Basin Native Plant Project: 2011 Progress Report. Boise, ID: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 133-134.
SEINet – Regional Networks of North American Herbaria Steering Committee [SEINet]. 2017. SEINet Regional Networks of North American Herbaria. https://symbiota.org/seinet
Shanklin, Amber. 2014. Experimental restoration treatments for burn pile fire scars in conifer forests of the Front Range, Colorado. Fort Collins, CO: Colorado State University. Thesis. 56 p.
Shaw, R.J. 1995. Utah wildflowers: A field guide to northern and central mountains and valleys. Logan, UT: Utah State University Press. 218 p.
Sherwood, J.A.; Debinski, D.M.; Caragea, P.C.; Germino, M.J. 2017. Effects of experimentally reduced snowpack and passive warming on montane meadow plant phenology and floral resources. Ecosphere. 8(3). doi:10.1002/ecs2.1745.
Shiflet, T.N. 1994. Rangeland cover types of the United States. Denver, CO: Society for Range Management. 152 p.
Shock, C.; Feibert, E.; Saunders, L.; Parris, C.; Shaw, N. 2011. Evaluation of herbicides for weed control in forb seed production. In: Shock, C.C., ed. Malheur Experiment Station Annual Report 2010. OSU AES Ext/CrS 132. Corvallis, OR: Oregon State University: 186-198.
Shock, C.C.; Feibert, E.B.G.; Rivera, A.; Saunders, L.D.; Shaw, N.; Kilkenny, F. 2018. Irrigation requirements for native buckwheat seed production in a semi-arid environment. In: Kilkenny, F; Edwards, F.; Irwin, J.; Barga, S., eds. Great Basin Native Plant Project: 2017 Progress Report. Boise, ID: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 110-118.
Shock, C.C.; Feibert, E.B.G.; Rivera, A.; Saunders, L.D.; Shaw, N.; Kilkenny, F.F. 2017. Irrigation requirements for seed production of two Eriogonum species in a semiarid environment. Hortscience. 52(9): 1188-1194.
Smith, A.D.; Beale, D.M. 1980. Antelope in Utah. No. 80-13. Salt Lake City, UT: Utah Division of Wildlife Resources. 88 p.
Smith, B.L. 1976. Ecology of Rocky Mountain goats in the Bitterroot Mountains, Montana. Missoula, MT: University of Montana. Thesis. 203 p.
Smith, N.J. 2008. A review of nearctic Ammoplanus Giraud 1869 (Hymenoptera: Crabronidae). The Pan-Pacific Entomologist. 84(4): 301-333.
St. John, L.; Ogle, D. 2011. Cooperative work between the Great Basin Native Plant Selection and Increase Project and the Aberdeen Plant Materials Center. In: Shaw, N.; Pellant, M., eds. Great Basin Native Plant Selection and Increase Project: 2010 Progress Report. Boise, ID: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 69-76.
Stevens, D.R. 1966. Range relationships of elk and livestock, Crow Creek Drainage, Montana. Journal of Wildlife Management. 30(2): 349-363.
Stevens, R.J.; Jorgensen, K.R.; Young, A.S.; Monsen, S.B. 1996. Forb and shrub seed production guide for Utah. AG 501. Logan, UT: Utah State University Extension. 52 p.
Steward, J.H. 1933. Ethnography of the Owens Valley Paiute. University of California Publications in American Archaeology and Ethnology. 33(3): 233-350.
Sutton, R.F.; Johnson, C.W. 1974. Landscape plants from Utah’s mountains. EC-368. Logan, UT: Utah State University, Cooperative Extension Service. 135 p.
Taylor, R.J. 1992. Sagebrush country: A wildflower sanctuary. Missoula, MT: Mountain Press Publishing Company. 211 p.
Tilley, D.; Bair, C. 2011. The Jet Harvester: A shop tool for harvesting forb and shrub seeds. Native Plants Journal. 12(2): 123-127.
Titus, J.H.; Tsuyuzaki, S. 1999. Ski slope vegetation of Mount Hood, Oregon, U.S.A. Arctic, Antarctic, and Alpine Research. 31(3): 283-292.
Tomback, D.F. 1982. Dispersal of whitebark pine seeds by Clark’s nutcracker: A mutualism hypothesis. Journal of Animal Ecology. 51(2): 451-467.
Train, P.; Henrichs, J.R.; Archer, W.A. 1941. Medicinal uses of plants by Indian tribes of Nevada: Part 1. Contributions toward a flora of Nevada. No. 33. Washington, DC: U.S. Department of Agriculture, Bureau of Plant Industry, Division of Plant Exploration and Introduction. 199 p.
Urza, A.K.; Weisberg, P.J.; Chambers, J.C.; Dhaemers, J.M.; Board, D. 2017. Post-fire vegetation response at the woodland-shrubland interface is mediated by the pre-fire community. Ecosphere. 8(6): e01851.
USDA Forest Service [USDA FS] 1937. Range plant handbook. Washington, DC: U.S. Department of Agriculture, Forest Service. 816 p.
USDA Forest Service, Bend Seed Extractory [USDA FS BSE]. 2017. Nursery Management Information System Version 4.1.11. Local Source Report 34-Source Received. Bend, OR: U.S. Department of Agriculture, Forest Service, Bend Seed Extractory.
USDA Forest Service, Great Basin Native Plant Project [USFS GBNPP]. 2014. Seed weight table calculations made in-house. Report on file. Boise, ID: U.S. Department of Agriculture, Forest Service, Boise Aquatic Sciences Laboratory. Available: https://www.fs.fed.us/rm/boise/research/shrub/Links/Seedweights.pdf
USDA Forest Service, Western Wildland Environmental Threat Assessment Center [USFS WWETAC]. 2017. TRM Seed Zone Applications. Prineville, OR: U.S. Department of Agriculture, Forest Service, Western Wildland Environmental Threat Assessment Center. https://www.fs.fed.us/wwetac/threat-map/TRMSeedZoneMapper.php
USDA Natural Resources Conservation Service [USDA NRCS]. 2017. The PLANTS Database. Greensboro, NC: U.S. Department of Agriculture, Natural Resources Conservation Service, National Plant Data Team. https://plants.usda.gov/java
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2016. Bureau of Land Management technical protocol for the collection, study, and conservation of seeds from native plant species for Seeds of Success. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program. 37 p.
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2017. Seeds of Success collection data. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program.
USDI Environmental Protection Agency [USDI EPA]. 2017. Ecoregions. Washington, DC: U.S. Department of the Interior, Environmental Protection Agency. https://www.epa.gov/eco-research/ecoregions
USDI Geological Survey [USDI USGS]. 2017. Biodiversity Information Serving Our Nation (BISON). U.S. Geological Survey. https://bison.usgs.gov/#home
Utah Crop Improvement Association [UCIA]. 2015. How to be a seed connoisseur. Logan, UT: UCIA, Utah Department of Agriculture and Food, Utah State University and Utah State Seed Laboratory. 16 p.
Utah State University Extension [USU Ext.] 2017. Range plants of Utah: Eriogonum umbellatum. Logan, UT: Utah State University. https://extension.usu.edu/rangeplants/forbsherbaceous/sulfur-buckwheat [Accessed 2019 April 4].
Walker, S.C.; Shaw, N.L. 2005. Current and potential use of broadleaf herbs for reestablishing native communities. In: Shaw, N.L.; Pellant, M.; Monsen, S.B., comps. Sage-grouse habitat restoration symposium proceedings; 2001 June 4-7; Boise, ID. RMRS-P-38. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 56-61.
Wall, M.; MacDonald, J. 2009. Processing seeds of California native plants for conservation, storage, and restoration. Claremont, CA: Rancho Santa Ana Botanic Garden. 216 p.
Welsh, S.L.; Atwood, N.D.; Goodrich, S.; Higgins, L.C., eds. 2015. A Utah Flora. Fifth Edition, revised. Provo, UT: Brigham Young University. 990 p.
West, N.E.; Tausch, R.J.; Tueller, P.T. 1998. A management-oriented classification of pinyon-juniper woodlands of the Great Basin. Gen. Tech. Rep. RMRS-GTR-12. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 42 p.
Whittaker, R.H. 1960. Vegetation of the Siskiyou Mountains, Oregon and California. Ecological Monographs. 30(3): 279-338.
Wiese, J.L.; Meadow, J.F.; Lapp, J.A. 2012. Seed weights for northern Rocky Mountain native plants with an emphasis on Glacier National Park. Native Plants Journal. 13(1): 39-49.
Woolley, S.B., comp. 1936. Root systems of important range plants of the Boise River watershed: A catalogue of species excavated by Liter E. Spence [collaborator]. Unpublished paper on file at: U.S. Department of Agriculture, Forest Service, Intermountain Fire Sciences Lab, Missoula, MT. p. 59.
Wright, R.D.; Mooney, H.A. 1965. Substrate-oriented distribution of bristlecone pine in the White Mountains of California. The American Midland Naturalist. 73(2): 257-284.
Wyman, L.C.; Harris, S.K. 1951. The ethnobotany of the Kayenta Navaho: An analysis of the John and Louisa Wetherill ethnobotanical collection. Publications in Biology. No. 5 Albuquerque, NM: University of New Mexico Press. 66 p.
Wynd, F.L. 1941. The botanical features of the Life Zones of Crater Lake National Park. The American Midland Naturalist. 25(2): 324-347.
Young, J.A. 1989. Germination of seeds of sulphur flower (Eriogonum umbellatum Torr.). Journal of Seed Technology. 13(1): 31-38.
Young, J.A.; Young, C.G. 1986. Collecting, processing and germinating seeds of wildland plants. Portland, OR: Timber Press. 236 p.
Young, S.A.; Schrumpf, B.; Amberson, E. 2003. The Association of Official Seed Certifying Agencies (AOSCA) native plant connection. Moline, IL: AOSCA. 9 p. Available: https://seedcert.oregonstate.edu/sites/seedcert.oregonstate.edu/files/pdfs/aoscanativeplantbrochure.pdf
Young-Matthews, A. 2012. Plant fact sheet: Sulphur-flower buckwheat (Eriogonum umbellatum) Torr. Corvallis, OR: U.S. Department of Agriculture, Natural Resources Conservation Service, Corvallis Plant Materials Center. 2 p.
Youngblood, A.; Metlen, K.L.; Coe, K. 2006. Changes in stand structure and composition after restoration treatments in low elevation dry forests of northeastern Oregon. Forest Ecology and Management. 234(1): 143-163.
Zigmond, M.L. 1981. Kawaiisu ethnobotany. Salt Lake City, UT: University of Utah Press. 102 p.
How to Cite
Gucker, Corey L.; Shaw, Nancy L. 2019. Sulphur-flower buckwheat (Eriogonum umbellatum). In: Gucker, C.L.; Shaw, N.L., eds. Western forbs: Biology, ecology, and use in restoration. Reno, NV: Great Basin Fire Science Exchange. Online:https://westernforbs.org/species/sulphur-flower-buckwheat-eriogonum-umbellatum/