Authorship
Gucker, Corey; Shaw, Nancy
Publication Date
March 2018
Update
May 2024
Nomenclature
Dieteria canescens (Pursh) Nutt. until recently (2010) was known as Machaeranthera canescens (Pursh) A. Gray (ITIS 2017; USDA NRCS 2024). This species belongs to the Astereae tribe of the Asteraceae family (Morgan 2006) and will hereafter be referred to by its common name, hoary tansyaster. The Lewis and Clark expedition collected a hoary tansyaster specimen somewhere along the Columbia River in October 1805 (Reveal et al. 1999).
Family
Asteraceae – Aster family
Genus
Dieteria
Species
canescens
NRCS Plant Code
MACA2 (USDA NRCS 2024).
Subtaxa
The Flora of North America (Morgan 2006) recognizes ten varieties of hoary tansyaster: Dieteria canescens var. canescens, ambigua, artistata, glabra, incana, leucanthemifolia, nebraskana, sessiliflora, shastensis, and ziegleri.
Synonyms
Dieteria canescens: Machaeranthera canescens (Pursh) A. Gray; Aster canescens Pursh
D. c. var. ambigua: M. canescens (Pursh) A. Gray var. ambigua B.L. Turner
D. c. var. aristatus: A. canescens Pursh var. aristatus Eastwood; M. canescens (Pursh) A. Gray var. aristata (Eastwood) B.L. Turner; M. rigida Greene
D. c. var. canescens: M. divaricata (Nuttall) Greene; M. laetevirens Greene; M. latifolia A. Nelson; M. pulverulenta (Nuttall) Greene; M. viscosa (Nuttall) Greene
D. c. var. glabra: M. canescens (Pursh) A. Gray var. glabra A. Gray; A. canescens Pursh var. viridis A. Gray; M. linearis Greene
D. c. var. incana: Diplopappus incanus Lindley; Dieteria incana (Lindley) Torrey & A. Gray; M. canescens (Pursh) A. Gray var. incana (Lindley) A. Gray; M. incana (Lindley) Greene
D. c. var. leucanthemifolia: A. leucanthemifolius Greene; M. canescens (Pursh) A. Gray var. leucanthemifolia (Greene) S.L. Welsh; M. leucanthemifolia (Greene) Greene
D. c. var. nebraskana: M. canescens (Pursh) A. Gray var. nebraskana B.L. Turner
D. c. var. sessiliflora: D. sessiliflora Nuttall; M. canescens (Pursh) A. Gray var. sessiliflora (Nuttall) B. L. Turner; M. sessiliflora (Nuttall) Greene
D. c. var. shastensis: M. shastensis A. Gray; A. shastensis (A. Gray) A. Gray; M. canescens (Pursh) A. Gray var. shastensis (A. Gray) B. L. Turner
D. c. var. ziegleri: M. canescens (Pursh) A. Gray subsp. ziegleri Munz; M. canescens var. ziegleri (Munz) B. L. Turner (Morgan 2006).
Common Names
Hoary tansyaster, hoary-aster, hoary goldenweed, hoary machaeranthera, Nebraska tansyaster, purple aster, rayless Shasta aster, Shasta tansyaster, small-leaf hoary aster, whiteflower tansyaster, Ziegler’s tansyaster (Welsh et al. 2015; Mansfield 2000; Morgan 2006; Tilley et al. 2014).
Chromosome Number
Chromosome number is 2n = 8 (Strother 1972; Anderson et al. 1974; Keil et al. 1988; Morgan 2006).
Hybridization
Intergradation is common where the distributions of Dieteria species and infrataxa meet or overlap. Variety canescens is the most widespread of the varieties and therefore has the greatest potential for forming hybrids. It intergrades with: D. bigelovii in Colorado, Utah, and Wyoming; variety ambigua in Utah; variety aristata in Utah and Colorado; variety glabra in Colorado; variety incana in Idaho and Washington; variety leucanthemifolia in Utah and California; variety nebraskana in Nebraska and South Dakota; variety sessiliflora in Idaho; variety shastensis in California, Nevada, and Oregon; and variety ziegleri in California (Morgan 2006; Hitchcock and Cronquist 2018).
Distribution
The species is common and broadly distributed throughout western North America, ranging from southern British Columbia south to Baja California and east to western North Dakota, western Texas, and Chihuahua, Mexico (Morgan 2006; Hitchcock and Cronquist 2018). Variety canescens is the most widespread of the varieties, most of which have much more limited distributions (Table 1; Morgan 2006).
Habitat And Plant Associations
Hoary tansyaster grows in a wide variety of community types throughout the western US where annual precipitation ranges from 8 to 60 in (200-1,520 mm) (Tilley et al. 2014), but it is most common in open, dry, low-elevation habitats (Hitchcock and Cronquist 2018).
Plants are found in semi-arid grasslands, meadows, shrublands, montane woodlands, and pine forests and often occur in gravelly or sandy soils of washes and along streams. Plant communities where hoary tansyaster often occurs include: sagebrush (Artemisia spp.), blackbrush (Coleogyne ramosissima), cold desert and saltbush (Atriplex spp.) scrub, greasewood-shadscale saltbush (Sarcobatus vermiculatus-A. confertifolia), creosote bush (Larrea tridentata), chaparral, mountain mahogany (Cercocarpus spp.), pinyon-juniper (Pinus–Juniperus spp.), pine-oak (Pinus–Quercus spp.), quaking aspen (Populus tremuloides)-sagebrush, Douglas-fir (Pseudotsuga menziesii), limber pine (Pinus flexilis), lodgepole pine (P. contorta), and ponderosa pine (P. ponderosa) (Flowers 1934; Munz and Keck 1973; Brotherson et al. 1984; Ehleringer 1988; Blackwell 2006; Morgan 2006; Ogle et al. 2012; Welsh et al. 2015).
In south-central Nevada’s Nye County where the Great Basin desert transitions into the Mojave desert, hoary tansyaster occurred in shadscale saltbush communities (Beatley 1976). In the Wasatch Mountains of central Utah, it was a prominent understory species of Gambel oak and Utah serviceberry (Amelanchier utahensis) woodlands (Yake and Brotherson 1979). On the North Rim of Grand Canyon National Park, Arizona, hoary tansyaster was common in ponderosa pine and ponderosa pine-Gambel oak (Q. gambelii) forests. It was considered a montane zone indicator when montane and subalpine communities were compared (Laughlin et al. 2005).
Elevation
Hoary tansyaster occupies sites from about sea level to 11,000 ft (0-3,400 m) (Table 1; Morgan 2006; Chambers 2020).
Soils
Hoary tansyaster grows well on medium- to coarse-textured soils with pH of 6 to 8.4 and rooting depths of at least 10 in (25 cm) (Tilley et al. 2014). It is commonly found in loamy to sandy alkaline soils with gravel and rocks (Blackwell 2006; EcoRestore AZ 2024), and it also grows in less optimal soil conditions.
Plants were found throughout 40 years of vegetation surveys of the Pumice Desert in Crater Lake National Park, Oregon, where soils were described as gravelly, ashy, loamy, coarse sands with excessive drainage and little profile development (Horn 2009). In northern Arizona ponderosa pine forests, hoary tansyaster was characteristic of xeric, loamy soils on low-nitrogen basalt sites (Abella et al. 2012). In Bingham County, Utah, frequency of hoary tansyaster was 50% on sulfide-bearing waste sites resulting from copper pit mining. Waste site age averaged 30 years, pH levels were neutral (6-7.9), and salinity levels were low (0.06-0.5 dS/m). Hoary tansyaster was absent from waste areas with lower pH and higher salinity (Borden and Black 2005). In Carter County, Montana, plants occurred with low cover on old bentonite mine spoils where pH averaged 5.6, sodium and sulphur concentrations were high, and compaction was extreme (average penetrometer reading: 480 kg/cm²) (Sieg et al. 1983).
Table 1. Distribution and elevational range for hoary tansyaster varieties (Morgan 2006; Chambers 2020).
|
Variety |
States/Provinces |
Elevation (ft) |
|
ambigua |
AZ, CO, NM |
4,300-8,500 |
|
aristata |
AZ, CO, NM, UT |
3,300-8,900 |
|
canescens |
AZ, CA, CO, ID, MT, ND, NE, NV, SD, UT, WY; Canada: AB, BC, SK |
330-9,800 |
|
glabra |
AZ, CO, KS, NM, TX, WY; Mexico: Chih. |
3,300-7,500 |
|
incana |
ID, OR, WA; Canada: BC |
0-4,900 |
|
leucanthemifolia |
CA, NV, UT |
1,600-8,200 |
|
nebraskana |
NE, SD |
3,300-4,900 |
|
sessiliflora |
ID (along the Snake River and its tributaries) |
2,000-5,900 |
|
shastensis |
CA, NV, OR (Cascade, Sierra Nevada, and adjacent mountains) |
1,300-11,000 |
|
ziegleri |
CA (Santa Rosa Mountains) |
4,600-8,200 |
Description
Hoary tansyaster is a highly variable species and grows as an annual, biennial, or short-lived perennial. The artistata and glabra varieties typically grow as annuals or biennials, variety nebraskana grows as a biennial or short-lived perennial, and variety ziegleri is a perennial subshrub (Morgan 2006; Hitchcock and Cronquist 2018).
Plants are taprooted, often with a simple to branched caudex producing one to many loosely spreading or erect, somewhat wiry, and unbranched or much branched stems (Fig. 1). Variety nebraskana is typically single stemmed. Plants are commonly 2.5 to 30 in (6-80 cm) tall, but robust specimens of up to 40 in (1 m) tall have been reported (Hitchcock et al. 1955; Morgan 2006; Welsh et al. 2015; Hitchcock and Cronquist 2018; Luna et al. 2018). Herbage is glabrous puberulent or canescent and occasionally stipitate-glandular (Hitchcock and Cronquist 2018). Hoary tansyaster flower heads, stems, and leaves are covered with a sticky, pungent resin (Pavek et al. 2012; Tilley et al. 2014). Leaves are alternate, fleshy and firm, sparsely to densely hairy, and linear to oblong. Leaf margins are entire to minutely toothed to toothed. Basal leaves, up to 4 in (10 cm) long and 0.8 in (2 cm) wide, are linear to oblong with dentate to entire margins and usually sessile. They may be persistent, shriveled, or absent at the time of flowering and seed production. Stem leaves are slightly smaller, sessile or petiolate, usually linear, and persistent (Mansfield 2000; Pavek et al. 2012; Welsh et al. 2015; Hitchcock and Cronquist 2018; Chambers 2020). Inflorescences are a well-branched panicle- or raceme-like array (Chambers 2020). Flower heads (1-1.5 in [2.5-3.8 cm] diameter) are numerous and produced singly or in simple flat- or round-topped inflorescences at branch ends (Fig. 2) (Hickman 1993; Andersen and Holmgren 1996; Morgan 2006; Welsh et al. 2015; Hitchcock and Cronquist 2018). The involucre is 5 to 11 mm tall with phyllaries in whorls of 4 to 10. Tips of the canescent to glandular-puberulent phyllaries are appressed to spreading or reflexed. (Hitchcock and Cronquist 2018; Chambers 2020). Flower heads contain 10 to many yellow disk flowers surrounded by 0 to 25 ray flowers, which are purple-blue to pink or white (Hickman 1993; Morgan 2006; Welsh et al. 2015; Hitchcock and Cronquist 2018; Chambers 2020). Disk flowers are bisexual with five erect, triangular corolla lobes (Chambers 2020). Ray flowers are pistillate and fertile, except for variety shastensis, which produces only sterile ray flowers (Hickman 1993; Morgan 2006). Fruits are very small achenes (cypselas), 2.5 to 4 mm long. Achenes have a dirty white, hair-like pappus of 40 to 50 bristles in whorls of one to three (Fig. 3; Welsh et al. 2015; Tilley 2015a; Hitchcock and Cronquist 2018). Achenes are oblanceolate to obovate, flattened and tapered toward the base (Chambers 2020). They are glabrous to sparsely strigose-pubescent and 8 to 12 ribbed. Throughout this review, the term seed will refer to the fruit (cypsela) and the seed it contains.
Plants growing along roadsides in central New Mexico produced an average of 121 flower heads per plant (range: 29-260). Heads averaged 32 ray flowers (range: 26-38) and 66 disk flowers (range: 54-80) (Parker and Root 1981).

Figure 1. Hoary tansyaster growing in New Mexico. Photo: USDI BLM NM930N SOS.
Descriptions of varieties.
ambigua: one to many stems with ascending branches; flowers with fertile ray florets; peduncles ≥ involucres that are 8-12 mm tall with phyllaries in whorls of 5 to 10, usually appressed, sometimes spreading (Morgan 2006).
aristata: one to many stems, flowers with fertile ray florets; peduncles ≥ involucres that are 6-10 mm tall with phyllaries in whorls of three to six, appressed, spreading, or reflexed (Morgan 2006).
canescens: one to several ascending stems with well developed leaves and eglandular hairs; flowers with fertile ray florets; peduncles ≥ involucres that are 6-10 mm tall with phyllaries in whorls of 5 to 10, spreading to reflexed (Mansfield 2000; Morgan 2006; Hitchcock and Cronquist 2018; Chambers 2020).
glabra: usually one erect stem with ascending branches; flowers with fertile ray florets; peduncles ≥ involucres that are 6-10 mm tall with phyllaries in whorls of four to eight, usually appressed, sometimes spreading, rarely reflexed (Morgan 2006).
incana: usually one stiff, erect stem with straight stiff branches; flowers with fertile ray florets; peduncles ≥ length of involucres that are 6-10 mm tall with phyllaries in whorls of 5 to 10, middle and lower having recurved tips (Morgan 2006; Hitchcock and Cronquist 2018; Chambers 2020).
leucanthemifolia: one or more stems with leaves reduced to bracts (<4 times long as wide) and usually with glandular hairs; peduncles ≥ involucres that are 6-10 mm tall with phyllaries in whorls of three to six, middle and lower ones usually having recurved tips (Mansfield 2000; Chambers 2020).
nebraskana: usually one stem; flowers with fertile ray florets; peduncles > length of involucres that are 10-15 mm tall with phyllaries in whorls of 5 to 10, reflexed (Morgan 2006).
sessilifolia: stems are densely stipitate-glandular; flower heads sessile or on peduncles ≤ length of involucres that are 6-10 mm tall with phyllaries in whorls of four to seven, spreading to reflexed (Morgan 2006; Hitchcock and Cronquist 2018).
shastensis: ascending to erect or rarely decumbent stems; flowers with no or sterile ray florets; phyllaries in whorls of mostly three to five, all erect or just lower series with recurved tips (Mansfield 2000; Morgan 2006; Hitchcock and Cronquist 2018; Chambers 2020).
ziegleri: usually >1 stem; flowers with fertile ray florets; peduncles ≥ involucres that are 12-16 mm tall with phyllaries in whorls of 5 to 10, reflexed (Morgan 2006).
Reproduction
Hoary tansyaster reproduces entirely from seed. Asexual reproduction does not occur.

Figure 2. Lower heads on hoary tansyaster plants contain 8 to 25 purple-blue to white ray flowers and ten or more yellow disk flowers. Photo: S. Murray, USDI BLM AZ932 SOS.

Figure 3. Hoary tansyaster seed collected from plants in Idaho. Photo: USDI BLM ID931 SOS.
Phenology
Plants flower from April to November (Andersen and Holmgren 1996; Blackwell 2006; Chambers 2020; LBJWC 2022) but are most common in summer and early fall (Andersen and Holmgren 1996). Plants flower in their first growing season. Flowering is indeterminate, and the first flowers appear in summer or fall depending on the variety and site conditions (Fig. 4) (Shaw et al. 2012; Tilley 2015b). There is a high degree of overlap in the timing of flowering among varieties (Table 2). However, varieties incana and leucanthemifolia are generally earliest, producing flowers in June. Flowers are commonly found into September for all varieties, and many produce flowers into October. Variety canescens can be found flowering into November (Morgan 2006).
Table 2. Hoary tansyaster varieties and their common flowering periods (Morgan 2006; Chambers 2020).
| Variety | Common flowering period |
| amigua | August-October |
| artistata | August-September |
| canescens | June-November |
| glabra | July-September |
| incana | May-October |
| leucanthemifolia | June-September |
| nebraskana | August-September |
| sessiliflora | August-October |
| shastensis | June-October |
| ziegleri | July-October |
Breeding System
Seed production. Seeds mature within 4 or 5 weeks of flowering (DeBolt and Parkinson 2005). The very small fruits (cypselas or false achenes) have a hair-like pappus (Welsh et al. 2015; Tilley 2015a; Hitchcock and Cronquist 2018) and are easily wind-dispersed (Drezner and Fall 2002).

Figure 4. Hoary tansyaster displaying indeterminate flowering in Idaho. Photo: USDI BLM ID931 SOS.
Pollination
Flowers attract a variety of pollinators and are considered a valuable late summer and early fall pollinator food source (Fig. 5) (Tilley et al. 2014). In some years, flowers are still present in mid-November, depending on the variety and weather conditions (Tilley 2015b). In seed production fields growing at the USDA NRCS Aberdeen Plant Materials Center in southern Idaho (IPMC), hoary tansyaster flowers were visited by various bees (Halictus spp., Agapostemon spp., and Apis mellifera), bee flies (Diptera: Bombyliidae), western white butterflies (Pontia occidentalis), and garden white butterflies (Pieris spp.) (Tilley et al. 2014, 2015b). Flowers are also pollinated by digger bees (Anthophora urbana, A. petrophila), western leafcutter bee (Megachile perihirta), confused long-horned bee (Melissoides confusa), and cinquefoil sweat bee (Duflourea harveyi) (Discover Life 2024).

Figure 5. Cabbage white butterfly (Pieris rapae) on hoary tansyaster flowers at the USDA NRCS Aberdeen Plant Materials Center in southern Idaho. Photo: D. Tilley, USDA NRCS.
Ecology
Hoary tansyaster is a fast-growing, early reproducing, disturbance-tolerant species.
Seed And Seedling Ecology
Hoary tansyaster is a prolific producer of wind-dispersed seed (Springer et al. 2022). Seed is generally considered non-dormant (see Germination Biology section) and although rare in seed bank studies it was recovered from soils collected for one such study. In a study that evaluated aboveground and belowground species composition in big sagebrush vegetation in northeastern Nevada, hoary tansyaster occurred in the aboveground vegetation at three plots, but did not emerge from soil samples collected at any of the 17 total study plots (Barga and Leger 2018). In another seedbank study, hoary tansyaster did not emerge from soils samples collected in late June in sagebrush shrublands of east-central Nevada but 707 seeds/m² were recovered from soil collected in early June in twoneedle pinyon-Utah juniper (Pinus edulis–Juniperus osteosperma) woodlands of the Colorado Plateau in southwestern Colorado. Seed bank composition in both studies was determined through greenhouse emergence trials (Hosna 2020).
Greenhouse and field research suggests that young hoary tansyaster seedlings are not competitive, especially with nonnative species (Parkinson et al. 2013; Prasser and Hild 2016; Tilley et al. 2020). In a competition study, hoary tansyaster (Amethyst germplasm, see Releases section) was grown alone, with one additional hoary tansyaster, and with low densities of cheatgrass. Seedlings were grown in 655 cc pots filled with Sunshine #4 media, irrigated as needed, and fertilized (24-8-16 NPK) 6 weeks after planting. The two species germinated within 7 days of each other. Hoary tansyaster seedling size decreased with competition from other hoary tansyaster and cheatgrass seedlings. At five weeks, hoary tansyaster seedlings grown without competition were large and grew more quickly than seedlings with competition. Plants grown with competition were noticeably stunted and occasionally died. At 12 weeks, a single additional hoary tansyaster plant reduced the volume of the target plant by 80% and a single cheatgrass plant decreased the volume by 95%. At 12 weeks, pots with a single additional hoary tansyaster plant reduced biomass of the target plant by 75% and a single cheatgrass plant reduced it by 93%. High-densities of cheatgrass (350-1400 plants/m²) reduced target plant volumes even more with average reductions of nearly 99% (Tilley et al. 2020).
In a greenhouse study, 12-week old hoary tansyaster plants weighed 3.4 g when grown alone, 5.3 g with Sanberg bluegrass (Poa secunda), and 5.5 g with bottlebrush squirreltail (Elymus elymoides), and 0.8 g with cheatgrass, the later was significantly less (P < 0.001) than when grown alone (Parkinson et al. 2013). In big sagebrush communities in north-central Oregon, hoary tansyaster abundance increased significantly 3 and 4 years following treatment with imazapic to control cheatgrass and increases occurred on 2 of 3 sites (Elseroad and Rudd 2011). In another greenhouse competition study, hoary tansyaster seedling growth (leaf number, plant height, root:shoot ratio, canopy area, and plant specific area) was not significantly different when grown as a monoculture or with saltlover (Halogeton glomeratus). However, survival of saltlover was 40% in monoculture but slightly lower, 30%, when grown with hoary tansyaster (Prasser and Hild 2016).
Disturbance Ecology
Successional status. Hoary tansyaster is an early colonizer in both primary and secondary succession (Brandt and Rickard 1994; Peinado et al. 2005; Pavek et al. 2012; Shaw et al. 2012; Stephens et al. 2016). It establishes readily from shattered seed (Tilley 2015b), and it is common on disturbed sites (Goodrich 1997; Loeser et al. 2007), and its abundance and persistence may benefit from periodic disturbance (Koniak and Everett 1982; Poreda and Wullstein 1994).
Hoary tansyaster commonly occurs in early seral, disturbed, and degraded communities (Ogle et al. 2012). It was a pioneer species on marble outcrops in the Sierra Nevada Mountains (Peinado et al. 2005) and on sandhills in Montana (Lesica and Cooper 1999) and Nebraska (Tolstead 1942). In sandhill vegetation in southwestern Montana, cover of hoary tansyaster was greatest (4.4%) in the earliest successional stage and decreased with advancing succession. Seral stages were primarily based on topography and sand movements with the earliest seral stage on lower slopes with active sand erosion, slightly later seral stages on upper slopes with active sand deposition, and late seral stages where sand was stabilized and shrub cover was greatest (Lesica and Cooper 1999).
Fire response. In several studies, hoary tansyaster appeared soon after fire. At The Nature Conservancy’s Boardman Grasslands Preserve in Morrow County, Oregon, hoary tansyaster was an indicator of burned sites (P < 0.05) (DiCarlo et al. 2019). The arid grassland and shrub-steppe vegetation (390-970 ft [120-295 m] elevation) supporting a mix of native and nonnative grasses burned in a moderate to severe surface fire in June 2015. Hoary tansyaster was more abundant the first year after the fire than before the fire (DiCarlo et al. 2019). Although not seeded, it occurred in the first, second, and third post-fire years following a summer fire and restoration treatments in sagebrush steppe in west-central Utah (Ott et al. 2003). Frequency of hoary tansy aster was 4% in the first and second and 2% in the third post-fire years on sites that were chained following seeding. Frequency was slightly higher, 7% in the first, 8% in the second, and 6% in the third post-fire years on plots that were not chained following seeding (Ott et al. 2003). In the central Wasatch Mountains near Midway, Utah, frequency was 9.8% on 1-year-old burns, but plants were absent from unburned big sagebrush sites (Poreda and Wullstein 1994). Hoary tansyaster occurred with 0.5% cover on 4-year-old burned mountain big sagebrush sites but was not found on adjacent unburned sites in Wasatch County, Utah (Goodrich 2006).
In ponderosa pine forests in northern and central Arizona, hoary tansyaster occurred on unburned, moderately burned, and high-severity burned plots evaluated 10 to 15 years after fires (Owen 2019). In ponderosa pine-Gambel oak habitats on the North Rim of Grand Canyon National Park, hoary tansyaster was an indicator species for burned sites. Sites were sampled 12 years after a low severity fire, and hoary tansyaster cover increased by at least 160% following burning (Springer et al. 2022).
On the North Rim of Grand Canyon National Park, Arizona, hoary tansyaster occurred in frequently burned old-growth ponderosa pine forests and in recently burned forests that were unburned for the previous 76 years. Hoary tansyaster was a significant indicator (P = 0.002) of frequently burned forests, where its relative abundance and frequency were high (Laughlin et al. 2004). In the Sweetwater Mountains of eastern California, hoary tansyaster was restricted to early seral sites when singleleaf pinyon (Pinus monophylla) woodlands ranging from recently burned and grass-forb dominated to late-seral, tree-dominated sites were compared. Hoary tansyaster was not found on shrub- or tree-dominated sites (Koniak and Everett 1982).
Wildlife And Livestock Use
Hoary tansyaster is a major range plant and provides excellent wildlife forage. It is an important component of sage-grouse habitat, supports many insects, and is an important pollinator species that attracts bees, butterflies, and moths (Lambert 2005; Ogle et al. 2012; Kartesz and BONAP 2014). Use of hoary tansyaster plants or seeds has been noted for elk (Cervus canadensis) (McCorquodale 1993), pronghorn (Antilocapra americana) (Beale and Smith 1970), Ord’s kangaroo rats (Dipodomys ordii) (Henderson 1990), packrats (Neotoma spp.) (Fisher et al. 2009), and greater sage-grouse (Centrocercus urophasianus) (Pyle 1993).
Large mammals. Heavy use of hoary tansyaster by elk and pronghorn has been reported. In big sagebrush-bluebunch wheatgrass (Pseudoroegneria spicata) communities in south-central Washington, the frequency of hoary tansyaster in elk diets as determined from fecal analyses was: 19.7% in October, 26% in November, 11.9% in December, 37.5% in January, 1.2% in February, and 0.3% in March (McCorquodale 1993). In the Greater Yellowstone area, mostly in Wyoming, elk use of stems and flowers was noted for July and November (Skinner 1928). In Bandelier National Monument, New Mexico, hoary tansyaster made up 0 to 2.9% of elk diets during periodic sampling from September 1991 to July 1993 (Wolters 1994).
Hoary tansyaster was moderately to highly preferred by pronghorn on the Desert Experimental Range in west-central Utah, even though its production reached a high of just 0.2 lb/ac (0.5 kg/ha). Plants were consumed more in wet than dry years based on pronghorn rumen samples collected between late August and early September (Beale and Smith 1970).
Small mammals. Seeds are eaten by Ord’s kangaroo rats (Henderson 1990) and vegetative material or seeds of hoary tansyaster were recovered from packrat middens in western North America (Thompson and Anderson 2000; Fisher et al. 2009). In the Mojave Desert, hoary tansyaster was identified as a microhabitat variable significantly associated with the relative abundance of desert kangaroo rats (D. deserti), but actual use of the species was not determined (Stevens and Tello 2009).
Birds. Hoary tansyaster attracts a variety of native insects that are important to certain life stages of greater sage-grouse and other birds (Tilley 2015b; Luna et al 2018), and it is an important component of greater sage-grouse habitats (Lambert 2005). At the Hart Mountain National Antelope Refuge in southeastern Oregon, frequency of hoary tansyaster was 2% in the diet of greater sage-grouse chicks (Pyle 1993).
Insects. Hoary tansyaster is a good nectar plant for butterflies (Tilley et al. 2019). The following lepidopterans utilize hoary tansyaster as a host: Charidryas acastus, Cucullia dorsalis, Dichomeris levisella, D. ochripalpella, Dichrorampha bittana, Eucosma bolanderana, Heliothis phloxiphaga, Oidaematophorus lacteodactylus, Phaneta alterana, and Schinia ligeae (Robinson 2024).
Livestock. Cattle did not appear to graze hoary tansyaster in a high-elevation, semi-arid grassland near Flagstaff, Arizona. Loeser et al. (2007) found no difference in the cover of hoary tansyaster in ungrazed, moderately grazed, and highly grazed pastures over an 8-year period.
Ethnobotany
Several western tribes report medicinal and ceremonial uses of hoary tansyaster. The Navajo dried the plant and used it as a snuff to treat nose and throat troubles (Elmore 1944). The Paiute also used it to treat throat problems, but they applied a poultice of leaves to swollen jaws or neck glands. Shoshoni Indians took a decoction of fresh or dried leaves for headaches. They used extracts of the whole plant for internal cleansing and a tea made from the plant to treat headaches (Train et al. 1941). Hopi Indians created a strong stimulant from an extract of the plant, which was given to women during childbirth (Whiting 1939). The Okanagan-Colville used hoary tansyaster in witchcraft ceremonies (Turner et al. 1980).
Current Medicinal Use
An evaluation of the phytochemistry of hoary tansyaster revealed that essential oil extracted from the plant was rich in both sesquiterpene hydrocarbons and oxygenated sesquiterpenoids, but relationships of these compounds to medicines were not indicated (Poudel et al. 2023).
Horticulture
Hoary tansyaster seed and plants are available commercially. It is highly valuable to wildlife and pollinators making it a good choice for use in campgrounds and other low maintenance natural landscapes. High establishment rates are common, and it tolerates disturbance. It grows well in loam, sand, and gravel soils and tolerates alkalinity (EcoRestore AZ 2024).
Revegetation Use
Many characteristics make hoary tansyaster a useful species for restoration, such as tolerance of early seral conditions; rapid establishment, growth, and reproduction; and food production for pollinators (Tilley et al. 2019). However, reports on its successful use in wildland restoration and rehabilitation are limited.
Although not planted on reclaimed or abandoned mine sites, hoary tansyaster occurred on bentonite mine spoils in Montana (Sieg et al. 1983) and copper mine waste sites in Utah (Borden and Black 2005). In Carter County, Montana, cover averaged 0.1% on old bentonite mine spoils, 0.2% on reclaimed spoils, and less than 0.1% in undisturbed big sagebrush-grassland communities (Sieg et al. 1983). In Bingham County, Utah, hoary tansyaster occurred at 50% frequency and up to 3% cover on sulfide-bearing waste created from copper pit mining. Waste sites averaged 30 years old and had neutral pH (6-7.9) and low salinity levels (0.06-0.5 dS/m). Hoary tansyaster was absent from waste sites with lower pH and higher salinity levels (Borden and Black 2005).
Developing A Seed Supply
For restoration to be successful, the right seed needs to be planted in the right place at the right time. Coordinated planning and cooperation is required among partners to first select appropriate species and seed sources and then properly collect, grow, certify, clean, store, and distribute seed for restoration (PCA 2015).
Developing a seed supply begins with seed collection from native stands. Collection sites are determined by current or projected revegetation requirements and goals. Production of nursery stock requires less seed than large-scale seeding operations, which may require establishment of agricultural seed production fields. Regardless of the size and complexity of any revegetation effort, seed certification is essential for tracking seed origin from collection through use (UCIA 2015).
Seed Sourcing
In an investigation of intraspecific functional trait variation using four hoary tansyaster populations (three from CO Plateau level III ecoregion (4,390-4,950 ft [1,340-1,510 m]) and one from AZ/NM Plateau ecoregion (6,680 ft [2,040 m]), within genotype trait differences accounted for the majority of variation in all traits. Variation in measured functional traits was often greater within populations than among populations. Populations closest in trait space came from different ecoregions. These findings reinforce the call for a more focused effort to manipulate trait richness rather than genotypic richness. For some species substantial trait variation can be obtained from collecting from genotypically diverse plants from a single population (Zeldin et al. 2020).
Because empirical seed zones are not currently available for hoary tansyaster, generalized provisional seed zones developed by Bower et al. (2014), may be used to select and deploy seed sources. These provisional seed zones identify areas of climatic similarity with comparable winter minimum temperature and aridity (annual heat:moisture index). In Figure 6, Omernik Level III Ecoregions (Omernik 1987) overlay the provisional seeds zones to identify climatically similar but ecologically different areas. For site-specific disturbance regimes and restoration objectives, seed collection locations within a seed zone and ecoregion may be further limited by elevation, soil type, or other factors. It is noteworthy that early findings of a study of the genetic variation of hoary tansyaster indicate that the species is divided into five genetic clusters that generally correspond to level III ecoregions in the Intermountain West (Kilkenny et al. 2022).
The Western Wildland Environmental Threat Assessment Center’s (USFS WWETAC 2024) Threat and Resource Mapping (TRM) Seed Zone application provides links to interactive mapping features useful for seed collection and deployment planning. The Climate Smart Restoration Tool (Richardson et al. 2020) can also guide restoration planning, seed collection, and seed deployment, particularly when addressing climate change considerations.
Occurrence Map
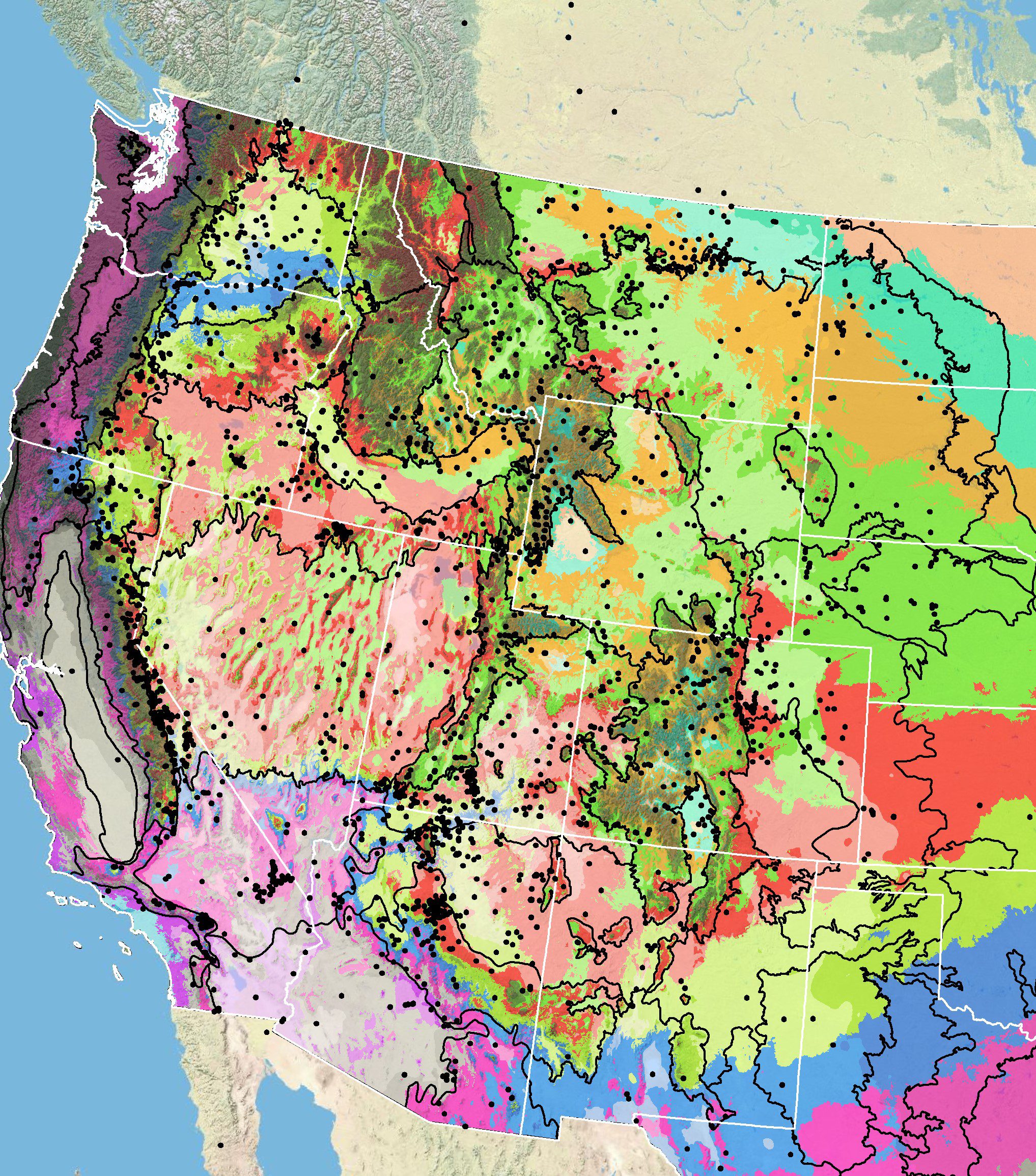
Figure 6. Distribution of hoary tansyaster (black circles) based on geo-referenced herbarium specimens and observational data from 1849-2016 (CPNWH 2017; SEINet 2017; USGS 2017). Generalized provisional seed zones (colored regions) (Bower et al. 2014) are overlain by Omernik Level III Ecoregions (black outlines) (Omernik 1987; USDI EPA 2018). Interactive maps, legends, and a mobile app are available (USFS WWETAC 2024). Map prepared by M. Fisk, USGS.
Releases
Amethyst Germplasm hoary tansyaster, a selected class natural track germplasm, was released for use in restoration in 2015. Amethyst Germplasm exhibited exceptional establishment, growth, and seed production in a common garden setting among populations collected from northern Utah and southern Idaho (Tilley 2015a). Amethyst came from seed collected at a site near the St. Anthony Sand Dunes in Freemont County, Idaho. At this source location, the elevation was 5,000 ft (1,500 m), soils were loamy fine sands, average annual precipitation was 12 in (300 mm), and dominant vegetation was antelope bitterbrush (Purshia tridentata), Indian ricegrass (Achnatherum hymenoides), and rubber rabbitbrush (Ericameria nauseosa) (Tilley 2014, 2015a).
Wildland Seed Collection
Although phenology can vary with variety, growing site, and weather conditions (e.g., elevation, aspect, moisture), it is typical to see mature hoary tansyaster seeds within 4 to 5 weeks of flowering (DeBolt and Parkinson 2005; Tilley 2015b). Because seed readily disarticulates when ripe and is wind dispersed, proper planning and timing of wildland seed harvests is critical to collecting large quantities of seed. Because flowering is indeterminate, several collections can be made over the ripening period (Fig. 7) (Tilley 2011, 2015b).
Wildland Seed Certification
Verification of species and tracking of geographic source is necessary whether wildland seed is collected for immediate project use or as stock seed for cultivated increase. This official Source Identification process can be accomplished by following procedures established by the Association of Official Seed Certifying Agencies (AOSCA) Pre-Variety Germplasm Program (UCIA 2015; Young et al. 2020). Wildland seed collectors should become acquainted with state certification agency procedures, regulations, and deadlines in the states where they collect.
If wildland-collected seed is to be sold for direct use in ecological restoration projects, collectors must apply for Source-Identified certification prior to making collections. Pre-collection applications, site inspections, and species and seed amount verification are handled by the AOSCA member state agency where seed collections will be made (see listings at AOSCA.org).
If wildland seed collected by a grower or private collector is to be used as stock seed for planting cultivated seed fields or for nursery propagation (See Agricultural Seed Field Certification section), detailed information regarding the collection site and collecting procedures must be provided when applying for certification. Photos and herbarium specimens may be required. Germplasm accessions acquired within established protocols of recognized public agencies, however, are normally eligible to enter the certification process as stock seed without routine certification agency site inspections. For contract grow-outs, however, this collection site information must be provided to the grower to enable certification.

Figure 7. Indeterminate flowering of hoary tansyaster in Oregon. Photo: USDI BLM OR931 SOS.
Collection Timing
Wildland seed collections of hoary tansyaster are most commonly made in late summer or early fall (USDI BLM SOS 2017, 2024). The BLM Seeds of Success (SOS) project provides records from 113 seed collections made from 2003 to 2022 in Washington (1), Wyoming (2), Colorado (5), New Mexico (5), California (7), Arizona (8), Oregon (15), Nevada (15), Idaho (21), and Utah (34). Seed was harvested most often in September (47) and October (37), but collections were also made in June (3), July (2), August (21), and November (3). Seed was collected at an earliest date of June 9, 2017 in Yavapai County, Arizona, at 4,077 ft (1,243 m) elevation. The latest collection date was November 9, 2015 in Santa Cruz County, Arizona, at 4,030 ft (13,222 m) elevation. In the single year with the most collections made (29 in 2018), the earliest harvest date was July 13 in Millard County, Utah, at 4,882 ft (1,488 m) and the latest was November 4 in Coconino County, Arizona, 4,801 ft (1,463 m). The majority of collection sites were revisited for additional harvests over time (USDI BLM SOS 2017, 2024). On the western side of the Cascades, seed can be collected as early as the end of August, and in Ontario, Oregon, collections can still be made in mid-October. These populations are thought to represent different hoary tansyaster varieties. The collection window lasts about 4 weeks. There are typically two to six ripe seed heads/plant near the beginning of the collection season, and this number increases a little as the collection season continues (B. Youtie, Eastern Oregon Stewardship Services [EOSS], personal communication, September 2017).
Collection Methods
Wildland seed can be collected by hand stripping or shaking branches over collection bags (Tilley 2011). Field crews for the USFS Great Basin Native Plant Project collected wildland seed by shaking seed from the stems or seed heads into paper bags. Because plants are sticky, gloves are recommended when handling stems and flower heads (A. Malcomb, USFS, personal communication, June 2017). Some prefer to hand-collect the puffs when seed heads have dry, brown flower petals and nearly mature seed (Fig. 8). The pappus generally emerges from flower material within 2 days of drying (B. Youtie, EOSS, personal communication, September 2017).
Several collection guidelines and methods should be followed to maximize the genetic diversity of wildland collections: 1) collect seed from a minimum of 50 randomly selected plants; 2) collect from widely separated individuals throughout a population without favoring the most robust or avoiding small stature plants; and 3) collect from all microsites including habitat edges (Basey et al. 2015). General collecting recommendations and guidelines are provided in online manuals (e.g., ENSCONET 2009; USDI BLM SOS 2023).
It is critical that wildland seed collection does not impact the sustainability of native plant populations. Collectors should take no more than 20% of the viable seed available at the time of harvest (USDI BLM SOS 2023). Additionally, care must be taken to avoid the inadvertent collection of weedy species, particularly those that produce seeds similar in shape and size to those of hoary tansyaster.

Figure 8. Collected hoary tansyaster seed heads. Photo: D. Wilson, Chicago Botanic Garden.
Post-Collection Management
Seeds collected late in the growing season or when conditions are cool and moist must be carefully dried before storing or cleaning. Seed should be kept in a dry, shaded place until collections can be moved to a controlled short-term storage environment. Short-term storage should be dry, cool, and inaccessible to rodents or other seed predators. If insects are suspected in any collection, seed should be frozen for 48 hours or treated with an appropriate insecticide. The more plant material in the collection, the more ventilation and drying a seed lot will likely need (Gold n.d.; Parkinson and DeBolt 2005; Hay and Probert 2011).
Seed Cleaning
Seed cleaning procedures depend on the harvest method and are more labor and time intensive for seed collections containing an abundance of plant material.
The following cleaning process was used by the USFS Bend Seed Extractory for a small lot of hand-collected seed (Barner 2009):
1. Process seeds using a Westrup Model LA-H brush machine, a number 30 mantel, and a medium rotation speed.
2. Air-screen the seed using an office Clipper, a 1/18 round-top screen, a blank bottom screen, and medium speed and air.
For seed collections containing flower heads and plant stems, the following seed cleaning guidelines were developed by the IPMC (Tilley et al. 2014):
1. Process flowers and seeds through a laboratory brush machine using a number 7 mantle and rotation speed of 3 with the gate closed. This breaks up the flower heads, disconnects seeds from their pappi, and allows seeds to fall into the catch pan.
2. Process brushed seed through a multi-deck, air screen cleaner using 6 × 30 and 6 × 32 screens and a low (1-2) air setting. This removes dust, any retained pappi, and unfilled seed. The air screening process may need to be repeated 6 to 10 times to adequately clean the seed.
3. Process screened seed through an indent cleaner, if needed, to remove weed seeds.
The cleaning procedures above can yield seed with 60 to 90% purity and viability (Tilley et al. 2014).
For field-grown seed collected using a vacuum (particularly the “jet harvester”; Tilley and Bair 2011), the IPMC developed the following cleaning guidelines (Tilley 2011):
1. Process seed through a Westrup brush machine with a number 7 mantle and a rotation speed of 2 with the gate closed to catch brushed seed. Seed lots may need to be brushed 2 to 3 times to remove seeds from the flower heads and to disconnect as many pappi as possible.
2. Clean brushed seeds with a Westrup LA-LS multi-deck air cleaner using a 2.3 top screen, blank middle screen, solid bottom screen, and a very low air setting (0.5) to remove remaining pappi and inert material.
Cleaning methods above for vacuum-collected seed, resulted in low seed purity (40-50%), but seed was clean enough to move through drill seeding equipment (Tilley 2011).
After more experimentation, researchers at the IPMC further reduced the vegetative material collected from seed fields (see Seed Harvesting section). This allowed development of simpler cleaning guidelines for seed lots containing primarily seeds, pappus fluff, and bracts (Simonson and Tilley 2016):
1. Process seed through a Westrup Laboratory Brush Machine using a number 7 mantle, keeping brushes 3 to 4 mm from the surface, setting the speed to 2, and leaving the gate open about 0.4 in (1 cm). Brushing the seed twice using these methods successfully removes the pappus from the seed.
2. Using the harvest and cleaning methods above, a single harvest on September 14 yielded 149 lbs (68 kg) of unclean and 27 lbs (12 kg) of clean seed (Simonson and Tilley 2016).
Seed Storage
Hoary tansyaster seeds are orthodox and survive drying to low moisture contents (SER, INSR, RBGK 2023). Seed was dried sufficiently in open collection sacks after 1 to 2 months at 50 °F (10 °C) and relative humidity of 20 to 30% (Tilley 2011, 2015a). Conditions for the best long-term seed storage were not described in the literature.
Seed Testing
There is no AOSA germination protocol available for hoary tansyaster. Guidelines for tetrazolium testing are available from Moore (1985). Seed is moistened for 6 to 18 hours, then cut longitudinally completely through the midsection of the distal half, and stained 6 to 24 hours. The embryo is exposed to evaluate staining by gently pressing the embryo through the distal opening and is viable if completely stained except for the distal third (Moore 1985).
Germination Biology
Seed produced by hoary tansyaster is mostly non-dormant and does not require cool-moist stratification to germinate. In field establishment studies where fall, spring, and summer seeding were evaluated, pre-chilling was not required for germination of seed collected from Idaho and Utah (Tilley et al. 2014). Germination of seed collected in the spring and summer from central New Mexico began within days of imbibition with or without stratification. Germination was 99 to 100% for untreated, cool-moist (41°F [5 °C]), and warm-moist (86 °F [30 °C]) stratified seed (Pendleton and Pendleton 2014). However, hoary tansyaster seed collected from San Juan County, New Mexico, germinated poorly when not exposed to cool-moist conditions (Kramer and Foxx 2016). Germination was 100% for seeds exposed to 12-hour light and 12-hour dark cycles for: 12 weeks at 34 °F (1 °C) and 4 weeks at alternating temperatures of 52 and 34 °F (11/1 °C), 59/41 (15/5 °C), or 68/50 °F (20/10 °C). Without initial pre-chilling, germination was 80% when seeds were incubated for 4 weeks at 52/35 °F (11/2 °C) and less than 25% when seeds were incubated for 4 weeks at 59/41 °F (15/5 °C) or for 4 weeks at 68/50 °F (20/10 °C) (Kramer and Foxx 2016).
Seed collected from the southern Idaho Snake River Plain, germinated within 10 to 14 days in a growth chamber after a single day of cool, moist stratification at 40 °F (4.4 °C) (Parkinson 2008). Seeds collected from Elmore County, Idaho, treated to cool-moist stratification for 35 days at 39 °F (4 °C) in the dark began germinating within 5 days in a germinator with 12-hr light/dark cycles at 70 °F (21 °C). After 8 days of incubation, germination was 76%, and total germination was 77% (DeBolt and Parkinson 2005). There were no reports of testing the germination of unchilled seed from either of the above studies.
Kildisheva et al. (2019) found hoary tansyaster to be nondormant and one of the fastest germinating species of 26 important dryland forbs and shrubs species important for restoration. Hoary tansyaster seed used in the study was collected in Crook County, Oregon (4,446 ft [1,355 m]). It germinated to at least 75% within 28 days at an incubation temperature of 59 °F (15 °C). Time to reach 50% germination was 11.8 days at an incubation temperature of 41 °F (5 °C). Time to 50% germination generally decreased with increasing temperatures and was 4.4 days at 68 °F (20 °C) and 5.9 days at 77 °F (25 °C) (Kildisheva et al. 2019).
Wildland Seed Yield And Quality
Post-cleaning seed yield and quality of seed lots collected in the Intermountain region are provided in Table 3 (USFS BSE 2017, 2023). The results indicate that hoary tansyaster seed can be cleaned to high levels of purity but that purity, seed fill, and viability of fresh seed are highly variable. Shock et al. (2014a, 2017a) reported viability of harvested seed from cultivated research plots ranged from 70 to 84%.
Hoary tansyaster seeds are tiny, averaging more than 1,100,000 seeds/lb (2,400,000 seeds/kg) (USFS BSE 2017, 2023). Other sources report a similar range of 521,000 to 2,160,000 seeds/lb (1,200,000-4,800,000 seeds/kg) (Ogle et al. 2011; Tilley 2011; Shock et al. 2014a, 2017a; USFS GBNPP 2017; SER, INSR, RBGK 2023). Seed lots with the pappus attached weighed about 3 lbs/bushel, and without the pappus weighed between 20 and 24 lbs/bushel (Tilley et al. 2014).
Table 3. Seed yield and quality of hoary tansyaster seed lots collected in the Intermountain region, cleaned and evaluated by the Bend Seed Extractory. Viability was tested by the Oregon State Seed Laboratory or the USFS National Seed Laboratory (USFS BSE 2017, 2023).
|
Characteristic |
Mean |
Range |
Samples (#) |
|
Bulk weight (lbs) |
0.87 |
0.04-13.35 |
121 |
|
Clean weight (lbs) |
0.10 |
0.001-2.86 |
121 |
|
Clean-out ratio |
0.15 |
0.003-0.83 |
121 |
|
Purity (%) |
91 |
30-99 |
121 |
|
Fill (%)¹ |
91 |
68-99 |
122 |
|
Viability (%)² |
89 |
49-99 |
94 |
|
Seeds/lb |
1,116,176 |
252,000-2,180,769 |
121 |
|
Pure live |
921,408 |
405,784-1,949,184 |
94 |
¹ 100 seed X-ray test
² Tetrazolium chloride test
Marketing Standards
Acceptable seed purity, viability, and germination specifications vary with revegetation plans. Purity needs are highest for precision seeding equipment like that used in nurseries, while some rangeland seeding equipment handles less clean seed quite well.
Agricultural Seed Production
Hoary tansyaster was grown over several years for seed production at two experimental sites, IPMC (Fig. 9) and Oregon State University’s Malheur Experiment Station (OSU MES). The IPMC farm is on the Snake River Plains at 4,300 ft (1,310 m) in Major Land Resource Area B11. Soils are Declo silt loams with a pH of 7.4 to 8.4. Annual precipitation averages 9.4 in (240 mm). Growing season length averages 110 days with the last spring frost in early June, and the first fall frost in mid-September. Temperatures range from highs of 98 °F (37 °C) to lows of -15 °F (-26 °C) (Tilley 2015b). At OSU MES, soils are Nyssa silt loams (Shock et al. 2017b), and annual precipitation averages 10 in (260 mm). Growing season averages 161 days with the last spring frost occurring between late March and May and the first fall frost between mid-September and late October. High and low temperatures can reach 102 °F (39 °C) and -9 °F (-23 °C) (Feibert and Shock 2017).
Hoary tansyaster grows rapidly (Ogle et al. 2011), produces harvestable seed crops after the first full growing season (Tilley 2011; Shaw et al. 2012), and for some ecotypes, 2 to 3 additional years of seed harvests are possible (Tilley 2011). Timing of planting impacts seed production. When planted in spring or early summer, hoary tansyaster does not produce a harvestable crop until the following growing season, and plants of some varieties die after second-season flowering and seed set (Tilley et al. 2014). Fields planted in late summer or fall will produce seed crops in the next two growing seasons (Tilley et al. 2014, 2015b). Cropping time was 4 months for production of seed (Tilley 2011).
Agricultural Seed Certification
In order to minimize genetic changes in specific accessions of native species when increased in cultivated fields, it is essential to track the geographic source and prevent inadvertent hybridization or selection pressure. This is accomplished by following third party seed certification protocols for Pre-Variety Germplasm (PVG) as established by the Association of Official Seed Certification Agencies (AOSCA). AOSCA members in the U.S., Canada, and other countries administer PVG requirements and standards that track the source and generation of planting stock. Field and cleaning facility inspections then monitor stand establishment, proper isolation distances, control of prohibited weeds, seed harvesting, cleaning, sampling, testing, and labeling for commercial sales ( UCIA 2015; Young et al. 2020).
Seed growers apply for certification of their production fields prior to planting and plant only certified stock seed of an allowed generation (usually less than four). The systematic and sequential tracking through the certification process requires preplanning, knowing state regulations and deadlines, and is most smoothly navigated by working closely with state certification agency personnel. See the Wildland Seed Certification section for more information on stock seed sourcing.
 Figure 9. Hoary tansyaster seed production at the USDA NRCS Aberdeen Plant Materials Center in southern Idaho. Photo: L. St. John, USDA NRCS.
Figure 9. Hoary tansyaster seed production at the USDA NRCS Aberdeen Plant Materials Center in southern Idaho. Photo: L. St. John, USDA NRCS.
Site Preparation
Two field systems were used successfully for hoary tansyaster seed production by the IPMC: planting in weed-barrier fabric and open drill-seeded fields. For both methods, fields were plowed, disked, and packed with a roller cultipacker before seeding (Tilley 2015b).
Seed Pretreatments
At OSU MES, hoary tansyaster was seeded on October 30, 2012, in Nyssa silt loam soils (pH 8.3 and 1.1% organic matter) at the experimental farm in Ontario, Oregon (Shock et al. 2019). It was seeded into 60-inch (152-cm) beds in rows 450 ft (137 m) long. Seed was placed on the soil surface using a custom small-plot grain drill at a rate of 20-30 seeds/ft of row. Seed was covered with thin layer of sawdust (2.6 oz/ft of row or 558 lb/acre). Beds were covered with row cover (N-sulate, DeWitt Co, Sikeston, MO) until March when it was replaced with bird netting on wire hoops (Shock et al. 2019). Pre-treating hoary tansyaster seeds with a liquid mix of fungicides did not improve stand or seed production (Shock et al. 2017a).
Weed Management
Hoary tansyaster establishes well with limited weed pressure. Seeding in late summer (August) when annual weed competition is low was optimal for seed production at the IPMC (Tilley 2015b). The use of weed barrier fabric can also limit competition from weeds in seed production fields (Tilley et al. 2014). Where weed barrier fabric is used, the establishment of low-growing perennial grasses at the fabric edges allows for the use of mowing for weed management. Mowing to control weeds means less cultivation and herbicide use, thus minimizing disturbances to ground-nesting pollinators (Tilley 2015b).
In trials conducted at OSU MES, hoary tansyaster showed some sensitivity to both pre- and post-emergence herbicides. However, stands were slightly better than untreated stands for 4 of 11 pre-emergent herbicides. Conversely, stand development was reduced in six of seven post-emergence herbicides tested. Application of a thiocarbamate pre-emergent herbicide resulted in the best stand development (Shock et al. 2014b).
Several weed species (e.g. sowthistle [Sonchus spp.] and prickly lettuce [Lactuca serriola]) produce seeds that are comparable in size, weight, and shape to those of hoary tansyaster and are difficult to remove in the seed cleaning process. These weeds should be controlled in the field through hand pulling or targeted chemical control (Tilley et al. 2014).
Seeding
Successful seed production crops of hoary tansyaster have been grown using 30- or 36-in (76 or 91 cm) row spacings in open fields (Tilley et al. 2014, 2017b) or 9- or 18-in (23 or 46 cm) plant spacings in single or double rows down the center of weed barrier fabric (Tilley et al. 2014; Tilley 2015b). Seeding rates of 0.35 to 1 lb PLS/ac (0.4-1.1 kg/ha) are recommended (Tilley et al. 2014; Simonson and Tilley 2016). The IPMC recommends using a drop tube manual seeder and a target of 5 to 20 seeds/hole for seeding in weed barrier fabric. The drop seeder used by the IPMC had a 3-in (7.6 cm) diameter tube with a spur at the bottom (Tilley 2015b).
Drill-seeding open fields using 36-in (91 cm)row spacings, a seeding rate of 0.35 lb PLS/ac (0.4 kg/ha), and a seeding depth of up to 0.25 in (0.6 cm), can provide for a target of 35 PLS/linear ft of row (Tilley 2015b). The rate for drill seeding and a target delivery of 45 to 50 PLS/ft² (480-540/m²) is 2 lbs PLS/ac (2.3 kg/ha). A diluent like rice hulls is recommended to improve seed flow through the drill equipment (Tilley et al. 2014).
Covering seeds after planting can improve stand productivity. At OSU MSE, the use of row cover improved hoary tansyaster stand growth and seed production more than other seed coverings, which included sawdust and sand. The row cover protected against soil drying and bird predation. The use of hydromulch when seeding hoary tansyaster was no better for stand growth and productivity than direct seeding without providing any protection (Shock et al. 2017a).
Establishment And Growth
Hoary tansyaster emerges in early spring (late April or early May) (Tilley 2011). Generalist bees active late in the growing season can be expected at hoary tansyaster flowers (J. Cane, USDA Agricultural Research Service, personal communication, November 2018). Plants grow and mature quickly, producing seed in late summer or fall. However, seed production depends on the timing of planting. If planted in the spring or early summer, little if any seed is produced, and some varieties may die after producing a single seed crop the second year. If planted in late summer or fall, plants produce seed the following summer or fall and at least one more seed crop is produced (Tilley et al. 2014; Tilley 2015b). Hoary tansyaster goes dormant after flowering in late summer or fall and tolerates mowing at that time (Tilley 2011).
When nine hoary tansyaster populations from Idaho and Utah were grown together at the IPMC, establishment ranged from 41 to 87%. Seeding occurred in November to allow for natural stratification (Tilley et al. 2014, 2015a). Hoary tansyaster establishment was good even with moderate weed competition (Tilley 2015b).
In a greenhouse study, the total relative growth rate of hoary tansyaster growing alone for a period of 12 weeks averaged 0.4 mg/mg/week, relative growth rate of shoots averaged 0.4 mg/mg/week and roots averaged 0.5 mg/mg/week (Parkinson 2008). The total biomass of hoary tansyaster averaged 0.4 g at 6 weeks, 1.1 g at 9 weeks, and 3.4 g at 12 weeks, and root-mass ratios were 0.2 at 6 weeks, and 0.2 at 9 weeks, and 0.3 at 12 weeks (Parkinson et al. 2013).
At OSU MES, flowering dates, irrigation dates, and harvests dates were as follows (Table 4, Shock et al. 2019).
Table 4. Dates of flowering, irrigation, and harvest for hoary tansyaster grown for seed production at the Oregon State University, Malheur Experiment Station farm near Ontario, Oregon.
| Year | Flowering | Irrigation | Harvest | |||
| Start | Peak | End | Start | End | ||
| 2013 | 8/13 | —- | 10/1 | 7/17 | 8/28 | 10/2 |
| 2014 | 8/20 | 9/17 | 10/5 | 7/22 | 9/2 | 10/6 |
| 2015 | 8/10 | 9/17 | 10/1 | 8/11 | 9/22 | 10/6, 10/15 |
| 2016 | 8/17 | 9/20 | 10/10 | —- | —- | Partial winter die off |
| 2017 | 8/29 | —- | 10/20 | —- | —- | —- |
| 2018 | 10/8 | —- | 10/22 | 8/23 | 9/20 | 10/22 |
—- no data were available.
Irrigation
Supplemental irrigation for seed production fields may not be necessary. For seed crops at the IPMC where annual rainfall ranges from 8 to 12 in (200-305 mm), plants growing in weed barrier fabric were not provided supplemental irrigation (Tilley et al. 2014). In irrigation trials at OSU MES that spanned 3 years (2013-2015), average seed yield of hoary tansyaster was greater but not significantly so in plots receiving 4 or 8 in (100-200 mm) of supplemental irrigation (Shock et al. 2017b). Seed yield for hoary tansyaster did not respond to irrigation (Table 5). Plants produced most seed in their second year (Shock et al. 2019).
Table 5. Seed yield of hoary tansyaster grown for seed production at Oregon State University, Malheur Experimental Station in Ontario, Oregon (Shock et al. 2019).
| Year* | 0 in | 4 in | 8 in |
| Seed yield (lbs/ac) | |||
| 2013 | 206a | 215a | 124b |
| 2014 | 946a | 1210a | 1026a |
| 2015 | 304a | 402a | 459a |
| 2018 | 330a | 426a | 381a |
*Winter and spring precipitation in 2013 was 3.2 inches lower than the 5-year average, in 2017 was 4.1 inches higher than average, and in all other years was near average. The accumulation of growing degree-days (50-86 °F [10-30 °C]) was higher than average from 2013 to 2016 and 2018 and close to average in 2017. Partial die off occurred in winter 2015-16, which resulted in stands too uneven for irrigation trials in 2016 and 2017. Natural reseeding occurred winter 2016-17, but plants did not flower until 2018 (Shock et al. 2019).
Pest Management
Hoary tansyaster is a host and/or food source for a variety of insects. It is the only confirmed host for a fruit fly (Neaspilota appendiculata), which feeds on its flower heads. In a study conducted in southern California, the fruit fly larvae damaged an average of 94% (range: 44-100%) of soft achenes after eggs of this insect were inserted into hoary tansyaster flower heads (Goeden 2000). Hoary tansyaster is also host to European aphids (Uroleucon erigeronense) (Jensen et al. 2010), scales (Acanthococcus cryptus) (Miller and Miller 1982), and sagebrush checkerspot butterflies (Chlosyne acastus) (Scott 1986 cited in Wahlberg 2001). The sagebrush checkerspot butterfly feeds on young leaf tissue (Cates 1980) and of 78 observations of the butterfly in the West, all were on hoary tansyaster (Cates 1981). In seed production plots at the IPMC, moth caterpillars (Cucillia spp.) fed on hoary tansyaster flowers but had little impact on plot-level seed production (Tilley et al. 2014).
In central New Mexico, hoary tansyaster populations were damaged by heavy predation from purple-striped grasshoppers (Hesperotettix viridis) (Parker and Root 1981). Researchers observed hoary tansyaster along roadsides but not within the interior broom snakeweed (Gutierrezia sarothrae)-grassland community. When researchers transplanted hoary tansyaster into the interior habitats, only protected plants survived. One-third of unprotected plants were colonized by purple-striped grasshoppers within 12 hours, and all plants were colonized within 3 days. On average, unprotected plants were completely defoliated within 7.5 days. Of the protected hoary tansyaster plants, 77% produced flowers. Purple-striped grasshoppers were found on hoary tansyaster roadside populations, but factors limiting herbivore damage there were unknown (Parker and Root 1981).
At OSU MES, hoary tansyaster crops were treated on July 1, 2013, with bifenthrin (19 oz/acre or 0.3 lb ai/acre) to control aphids (Shock et al. 2019). Hoary tansyaster was also found to be a host for powdery mildew (Leveillula picridis) (Braun and Mohan 2013). In wild populations in Utah, rust (Puccina asteris) was found on hoary tansyaster leaves (Garrett 1910). The fungal infections were not discussed in terms of plant or seed production.
Seed Harvesting
Seed can be harvested by hand, vacuuming, combining, or by swathing and windrowing plants (Fig. 10). The method used will depend on resources, desire for multiple harvests, and/or minimizing plant damage (Tilley et al. 2014). Seed matures 4 to 5 weeks after flowering and is typically harvested in late summer or fall (DeBolt and Parkinson 2005; Tilley et al. 2014; Shock et al. 2017b) depending on variety and location. In seed fields at IPMC, plants began flowering in late summer and continued flowering for several weeks into the fall. Only hand harvesting and vacuum harvesters allowed for multiple non-destructive harvests (Tilley et al. 2014). In seed production fields at OSU MES, the earliest first flowering date was August 10 and latest last flowering date was October 22 in 6 years of observations (Shock et al. 2019).

Figure 10. Hoary tansyaster harvested using a standard Flail-Vac and containing significant amounts of vegetative material. Photo: D. Tilley, USDA NRCS.
Because hoary tansyaster flowers indeterminately, seed can be harvested multiple times using non-destructive hand or vacuum harvesting methods (Tilley et al. 2014). When growing on weed barrier fabric, the IPMC harvested hoary tansyaster using a “Jet Harvester” (see Tilley and Bair 2011) with a fan speed of 1,900 rpm. This harvest method allowed for multiple harvests of only ripe seed and provided seed lots with limited trash and other inert material. Harvesting seed from both sides of a 500-ft (150 m) row took about 90 minutes. Typically, three to five harvests were made over the season. Collected seed was air dried with the help of fans and manual turning of the seed twice a day (Tilley 2015b).
Researchers at the IPMC developed a unique non-destructive seed collection method for larger seed fields than those discussed above through modification of a flail-vacuum harvester (Simonson and Tilley 2016). The equipment modification added a system of loose chains to the flail-vacuum hood, which agitated plants and dislodged ripe seed from the flower heads. This produced limited plant damage, allowed for repeat harvests, and collected a minimal amount of vegetative material in the harvest. For a single harvest on September 14, this collection method yielded 149 lbs (68 kg) of bulk seed or 27 lbs (12 kg) of clean seed. Harvested material was primarily seed, pappus fluff, and bracts from the flower heads (Simonson and Tilley 2016). Seed cleaning of these harvests was much quicker and simpler than seed collected by destructive harvest methods (see Seed Cleaning section). This method could also be used for other species with indeterminate, lightweight seed produced throughout the plant rather than at the periphery of the canopy.
Seed Yields And Stand Life
At OSU MES, hoary tansyaster seed was harvested from small research plots by cutting and windrowing plants. After plants dried for 2 days, they were manually beat against plastic tubs to separate the seed heads from the flower stalks. Seed yields for this single destructive harvest ranged from 200 to 400 lbs of seed/ac (225-450 kg/ha) (Shock et al. 2017b).
Nursery Practice
A single report described procedures for small-scale production of hoary tansyaster nursery stock (DeBolt and Barrash 2013). Plants were grown from seed collected southeast of Boise, Idaho. Three to five seeds with pappi were planted into 2.9 x 5.5-in (7.3 x 14 cm) containers filled with a 2:1:5 mix of lava fines, perlite, and nursery soil mix. Seeds were planted on February 27, covered with a 0.13-in (0.33 cm) layer of chicken grit, kept outdoors in full sun, and hand watered as necessary. No fertilizers were applied. First emergence occurred on March 16. By April 3, about 80% of the containers had at least one seedling. Container stock was outplanted in early October. Survival of outplanted stock was not reported (DeBolt and Barrash 2013).
Hoary tansyaster grows quickly (DeBolt and Parkinson 2005; Ogle et al. 2011). Seeds germinate within about 5 days of exposure to incubation conditions and produce secondary leaves within about 1 week of germination. If seeds are pregerminated prior to planting, use of soil in germination dishes is recommended because roots quickly penetrate germination blotters (DeBolt and Parkinson 2005). In the production of hoary tansyaster container stock, the time to establishment was about 2 months and the time to produce roots that filled the container was about 6 months (DeBolt and Barrash 2013).
Although hoary tansyaster is host to several potential pests (see Pest Management section), the degree to which these might be problematic in nursery or crop systems is unknown.
Wildland Seeding And Planting
Based on seed production trials conducted by the IPMC, broadcast- or drill-seeding in the late fall into a firm, weed-free seed bed at sites receiving 7 to 15 in (180–380 mm) of precipitation is recommended (Ogle et al. 2011; Tilley et al. 2014, 2015b). Seeding depth should be 0 to 0.25 in (0.6 cm) (Tilley 2015b), and the recommended pure stand seeding rate is 1 to 2 lbs of PLS/ac (1.1-2.3 kg PLS/ha) for pure stands (Ogle et al. 2011; Tilley et al. 2014) or a target delivery of 20 to 30 PLS/ft² (215-323 PLS/m²). Broadcast seeding rates are generally double that of drill seeding rates, and broadcast seeding should be followed by some method that ensures good seed-soil contact. For drill seeding, a dilutent like rice hulls improves seed flow through the equipment (Tilley et al. 2014).
Often hoary tansyaster is just one component of a wildland seed mix and normally makes up less than 10% of the mixture (Ogle et al. 2012). Hoary tansyaster makes a good candidate for inclusion in pollinator and wildlife rangeland seed mixes. It has been observed to germinate after late summer and fall rains and is common in early-seral and disturbed sites (Tilley et al. 2020).
Hoary tansyaster emergence was too low to allow for any analysis in Utah and Nevada, where effects of increasing the seeding depth and adding row cover (N-sulate (DeWitt Co, Sikeston, MO) on emergence and survival were tested. Researchers suggested that the species may not be appropriate or emerge successfully when drill seeded and establishment might be improved by broadcast seeding followed by imprinting (Jensen et al. 2022).
Acknowledgements
Funding for Western Forbs: Biology, Ecology, and Use in Restoration was provided by the USDI BLM Great Basin Native Plant Materials Ecoregional Program through the Great Basin Fire Science Exchange. Great thanks to the chapter reviewers: Jim Cane, USDA ARS and Siri Jackman, Oregon Wildland Plant Resources.
This research was supported in part by the USDA Forest Service, Rocky Mountain Research Station. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA or U.S. Government determination or policy.
Literature Cited
Abella, S.R.; Cayenne Engel, E.; Springer, J.D.; Wallace Covington, W. 2012. Relationships of exotic plant communities with native vegetation, environmental factors, disturbance, and landscape ecosystems of Pinus ponderosa forests, USA. Forest Ecology and Management. 271: 65-74.
Andersen, B.A.; Holmgren, A.H. 1996. Mountain plants of northeastern Utah. HG 506. Logan, UT: Utah State University. 124 p.
Anderson, L.C.; Kyhos, D.W.; Mosquin, T.; Powell, A.M.; Raven, P.H. 1974. Chromosome numbers in Compositae. IX. Haplopappus and other Astereae. American Journal of Botany. 61(6): 665-671.
Barga, S.; Leger, E.A. 2018. Shrub cover and fire history predict seed bank composition in Great Basin shrublands. Journal of Arid Environments. 154: 40-50.
Barner, J. 2009. Propagation protocol for production of propagules (seeds, cuttings, poles, etc.) Machaeranthera canescens (Pursh) A. Gray seeds. Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://npn.rngr.net/propagation/protocols [Accessed 2017 July 21].
Basey, A.C.; Fant, J.B.; Kramer, A.T. 2015. Producing native plant materials for restoration: 10 rules to collect and maintain genetic diversity. Native Plants Journal. 16(1): 37-53.
Beale, D.M.; Smith, A.D. 1970. Forage use, water consumption, and productivity of pronghorn antelope in western Utah. The Journal of Wildlife Management. 34(3): 570-582.
Beatley, J.C. 1976. Vascular plants of the Nevada Test Site and central-southern Nevada: Ecologic and geographic distributions. TID-26881. Washington, DC: Technical Information Center, Energy Research and Development Administration. 306 p.
Blackwell, L.R. 2006. Great Basin wildflowers: A guide to common wildflowers of the high deserts of Nevada, Utah, and Oregon. Helena, MT: Morris Book Publishing. 288 p.
Borden, R.; Black, R. 2005. Volunteer revegetation of waste rock surfaces at the Bingham Canyon Mine, Utah. Journal of Environmental Quality. 34: 2234-2242.
Bower, A.D.; St. Clair, J.B.; Erickson, V. 2014. Generalized provisional seed zones for native plants. Ecological Applications. 24(5): 913-919.
Brandt, C.A.; Rickard, W.H. 1994. Alien taxa in the North American shrub-steppe four decades after cessation of livestock grazing and cultivation agriculture. Biological Conservation. 68(2): 95-105.
Braun, U.; Mohan, S.K. 2013. New records and new host plants of powdery mildews (Erysiphales) from Idaho and Oregon (USA). Schlechtendalia. 27: 7-10.
Brotherson, J.D.; Anderson, D.L.; Szyska, L.A. 1984. Habitat relations of Cercocarpus montanus (true mountain mahogany) in central Utah. Journal of Range Management. 37(4): 321-324.
Cates, R.G. 1980. Feeding patterns of monophagous, oligophagous, and polyphagous insect herbivores: The effect of resource abundance and plant chemistry. Oecologia. 46(1): 22-31.
Cates, R.G. 1981. Host plant predictability and the feeding patterns of monophagous, oligophagous, and polyphagous insect herbivores. Oecologia. 48(3): 319-326.
Consortium of Pacific Northwest Herbaria [CPNWH]. 2017. Agoseris aurantiaca. Seattle, WA: University of Washington Herbarium, Burke Museum of Natural History and Culture. http://www.pnwherbaria.org/ [Accessed 2020 October 25].
Cronquist, A. 1955. Part 5: Compositae. In: Hitchcock, C.L.; Cronquist, A.; Ownbey, M.; Thompson, J.W. Vascular plants of the Pacific Northwest. Seattle, WA: University of Washington Press. 343 p.
DeBolt, A.; Parkinson, H. 2005. Propagation protocol for production of container (plug) Machaerathera canescens (Pursh) A. Gray plants 2.875-inch x 5.5-inch plant band (container). Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://NativePlantNetwork.org [Accessed 2017 July 21].
DeBolt, A.M.; Barrash, K. 2013. Propagation protocol for production of container (plug) Machaerathera canescens (Pursh) A. Gray plants 2.875-inch x 5.5-inch plant band (container). Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://NativePlantNetwork.org [Accessed 2017 July 21].
DiCarlo, L.A.S.; DeBano, S.J.; Burrows, S. 2019. Short-term response of two beneficial invertebrate groups to wildfire in an arid grassland system, United States. Rangeland Ecology and Management. 72(3): 551-560.
Drezner, T.D.; Fall, P.L. 2002. Effects of inter-annual precipitation patterns on plant cover according to dispersal mechanisms along a riparian corridor in the Sonoran Desert, U.S.A. Journal of the Arizona-Nevada Academy of Science. 34(2): 70-80.
EcoRestore Portal: Restore Arizona’s Native Plants [EcoRestore AZ]. 2024. University of Arizona. Available: https://ecorestore.arizona.edu/
Ehleringer, J.R. 1988. Changes in leaf characteristics of species along elevational gradients in the Wasatch Front, Utah. American Journal of Botany. 75(5): 680-689.
Elmore, F.H. 1944. Ethnobotany of the Navajo: A monograph of the University of New Mexico and the School of American Research No. 8. Santa Fe, NM: University of New Mexico. 136 p.
Elseroad, A.C.; Rudd, N.T. 2011. Can imazapic increase native species abundance in cheatgrass (Bromus tectorum) invaded native plant communities? Rangeland Ecology & Management 64(6): 641-648.
European Native Seed Conservation Network [ENSCONET]. 2009. ENSCONET seed collecting manual for wild species. Edition 1: 32 p.
Feibert, E.B.G.; Shock, C.C. 2017. 2016 weather report. In: Shock, C.C., ed. Malheur Experiment Station Annual Report 2016. OSU AES Ext/CrS157. Corvallis, OR: 1-10.
Fisher, J.; Cole, K.L.; Anderson, R.S. 2009. Using packrat middens to assess grazing effects on vegetation change. Journal of Arid Environments. 73(10): 937-948.
Flowers, S. 1934. Vegetation of the Great Salt Lake region. Botanical Gazette. 95(3): 353-418.
Garrett, A.O. 1910. The smuts and rusts of Utah. Mycologia. 2(6): 265-304.
Goeden, R.D. 2000. Life history and description of immature stages of Neaspilota appendiculata Freidberg and Mathis (Diptera:Tephritidae) on Machaeranthera canescens (Pursh) A. Gray (Asteraceae) in southern California. Proceedings of the Entomological Society of Washington. 102(3): 519-532.
Gold, K. n.d. Post-harvest handling of seed collections. Technical Information Sheet 04. UK: Royal Botanic Gardens Kew and Millennium Seed Bank Partnership. 4 p.
Goodrich, S. 1997. Multiple use management based on diversity of capabilities and values within pinyon-juniper woodlands. In: Monsen, S.B.; Stevens, R., eds. Ecology and management of pinyon-juniper communities within the Interior West: Sustaining and restoring a diverse ecosystem. Provo, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 164-171.
Goodrich, S. 2008. Trout Creek 1999 burn. In: Kitchen, S.G.; Pendleton, R.L.; Monaco, T.A.; Vernon, J., comps. Proceedings- Shrublands under fire: Disturbance and recovery in a changing world. 2006 June 6-8; Cedar City, UT. Proc. RMRS-P-52. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 147-150.
Hay F.R.; Probert, R.J. 2011. Chapter 20: Collecting and handling seeds in the field. In: Guarino, L.; Ramanatha, V.; Goldberg, E. Collecting plant genetic diversity: Technical Guidelines-2011 update. Rome, Italy: Bioversity International. 33 p.
Henderson, C.B. 1990. The influence of seed apparency, nutrient content and chemical defenses on dietary preference in Dipodomys ordii. Oecologia. 82(3): 333-341.
Hickman, J.C., ed. 1993. The Jepson manual: Higher plants of California. Berkeley, CA: University of California Press. 1400 p.
Hitchcock, C.L.; Cronquist, A. 2018. Flora of the Pacific Northwest: An illustrated manual. Second Ed. Giblin, D.E.; Legler, B.S.; Zika, P.F.; Olmstead, R.G., eds. Seattle, WA: University of Washington Press. 882 p.
Hitchcock, C.L.; Cronquist, A.; Ownbey, M.; Thompson, J.W. 1955. Vascular plants of the Pacific Northwest, Part 5: Compositae. Seattle, WA: University of Washington Press. 510 p.
Horn, E.L. 2009. Forty years of vegetation changes on the Pumice Desert, Crater Lake National Park, Oregon. Northwest Science. 83(3): 200-210.
Hosna, R.K. 2020. Legacies of fire and presence of woody vegetation on soil seed banks of four North American desert systems. Las Cruces, NM: New Mexico State University. Thesis. 46 p.
Integrated Taxonomic Information Database [ITIS]. 2017. The Integrated Taxonomic Information System on-line database, http://www.itis.gov [Accessed 2017 September].
Jensen, A.S.; Miller, G.L.; Carmichael, A. 2010. Host range and biology of Uroleucon (Lambersius) erigeronense (Thomas 1878), and its synonymy with Uroleucon (Lambersius) escalantii (Knowlton) 1928 (Hemiptera: Aphididae). Proceedings of the Entomological Society of Washington. 112(2): 239-245.
Kartesz, J.T.; The Biota of North America Program (BONAP). 2015. North American Plant Atlas. http://bonap.net/napa
Keil, D.J.; Luckow, M.A.; Pinkava, D.J. 1988. Chromosome studies in Asteraceae from the United States, Mexico, the West Indies, and South America. American Journal of Botany. 75(5): 652-668.
Kildisheva, O.A.; Erickson, T.E.; Madsen, M.D.; Dixon, K.W.; Merritt, D.J. 2019. Seed germination and dormancy traits of forbs and shrubs important for restoration of North American dryland ecosystems. Plant Biology. 21: 458-469.
Kilkenny, F.; Barga, S.; Irwin, J.; Riskowsky, K.; Richardson, B.; Dumroese, K.; Massatti, R.; Winkler, D.; Bradford, J.; Leger, B.; Bartz, T.; Kulmatiski, A.; Aaronson, J.; Baughman, O. 2022. Workhorse native forb adaptive genetics and plant materials development for postfire restoration of critical wildlife habitat. In: Great Basin Native Plant Project Report 2018-2021. Boise, ID: U.S. Department of Agriculture, Rocky Mountain Research Station: 4-12.
Koniak, S.; Everett, R.L. 1982. Seed reserves in soils of successional stages of pinyon woodlands. The American Midland Naturalist. 108(2): 295-303.
Kramer, A.; Foxx, A. 2016. Propagation protocol for production of propagules (seeds, cuttings, poles, etc.) Machaeranthera canescens (Pursh) A. Gray seeds. Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://NativePlantNetwork.org [Accessed 2017 July 21].
Lady Bird Johnson Wildflower Center [LBJWC]. 2022. Machaeranthera canescens (Pursh) A. Gray. Native Plant Database. Austin, TX: Lady Bird Johnson Wildflower Center, The University of Texas at Austin. https://www.wildflower.org/plants-main [Accessed 2024 March 15].
Lambert, S. 2005. Guidebook to the seeds of native and non-native grasses, forbs and shrubs of the Great Basin. Boise, ID: U.S. Department of the Interior, Bureau of Land Management, Idaho State Office. 136 p.
Laughlin, D.C.; Bakker, J.D.; Fulé; P.Z. 2005. Understorey plant community structure in lower montane and subalpine forests, Grand Canyon National Park, USA. Journal of Biogeography. 32(12): 2083-2102.
Laughlin, D.C.; Bakker, J.D.; Stoddard, M.T.; Daniels, M.L.; Springer, J.D.; Gildar, C.N.; Green, A.M.; Covington, W.W. 2004. Toward reference conditions: Wildfire effects on flora in an old-growth ponderosa pine forest. Forest Ecology and Management. 199(1): 137-152.
Lesica, P.; Cooper, S.V. 1999. Succession and disturbance in sandhills vegetation: Constructing models for managing biological diversity. Conservation Biology. 13(2): 293-302.
Loeser, M.R.; Sisk, T.D.; Crews, T.E. 2007. Impact of grazing intensity during drought in an Arizona grassland. Conservation Biology. 21(1): 87-97.
Luna, T.; Mousseaux, M.R.; Dumroese, R.K. 2018. Common native forbs of the northern Great Basin important for greater sage-grouse. Gen. Tech. Rep. RMRS-GTR-387. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station; Portland, OR: U.S. Department of the Interior, Bureau of Land Management, Oregon-Washington Region. 76 p.
Mansfield, D.H. 2000. Flora of Steens Mountain. Corvallis, OR: Oregon State University Press. 410 p.
McCorquodale, S.M. 1993. Winter foraging behavior of elk in the shrub-steppe of Washington. The Journal of Wildlife Management. 57(4): 881-890.
Miller, D.R.; Miller, G.L. 1982. Systematic analysis of Acanthococcus (Homoptera: Coccoidea: Eriococcidae) in the western United States. Transactions of the American Entomological Society. 118(1): 1-106.
Moore, R.P. 1985. Handbook on tetrazolium testing. Zurich, Switzerland: International Seed Testing Association. 99 p.
Morgan, D.R. 2006. 197. Dieteria. In: Flora of North America Editorial Committee, ed. Flora of North America North of Mexico. Volume 20 Magnoliophyta: Asteridae, part 7: Asteraceae, part 2 Asterales, part 2 (Aster order). New York, NY: Oxford University Press: 395-401.
Munz, P.A.; Keck, D.D. 1973. A California flora and supplement. Berkeley, CA: University of California Press. 1905 p.
Ogle, D.; St. John, L.; Stannard, M.; Holzworth, L. 2012. Conservation plant species for the Intermountain West. Plant Materials Technical Note 24. Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 57 p.
Ogle, D.; Tilley, D.; Cane, J.; St. John, L.; Fullen, K.; Stannard, M.; Pavek, P. 2011. Plants for pollinators in the Intermountain West. Plant Materials Tech. Note 2A. Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 40 p.
Omernik, J.M. 1987. Ecoregions of the conterminous United States. Map (scale 1:7,500,000). Annals of the Association of American Geographers. 77(1): 118-125.
Ott, J.; McArthur; E.D.; Roundy, B. 2003. Vegetation of chained and non-chained seedings after wildfire in Utah. Journal of Range Management. 56: 81-91.
Parker, M.A.; Root, R.B. 1981. Insect herbivores limit habitat distribution of a native composite, Machaeranthera canescens. Ecology. 62(5): 1390-1392.
Parkinson, H.; DeBolt, A. 2005. Propagation protocol for production of container (plug) Chaenactis douglasii (Hook.) Hook. & Arn. plants. Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://NativePlantNetwork.org [Accessed 2017 April 23].
Parkinson, H.; Zabinski, C.; Shaw, N. 2013. Impact of native grasses and cheatgrass (Bromus tectorum) on Great Basin forb seedling growth. Rangeland Ecology and Management. 66(2): 174-180.
Parkinson, H.A. 2008. Impacts of native grasses and cheatgrass on Great Basin forb development. Bozeman, MT: Montana State University. Thesis. 73 p.
Pavek, P.; Erhardt, B.; Heekin, T.; Old, R. 2012. Forb seedling identification guide for the Inland Northwest: Native, introduced, invasive, and noxious species. Pullman, WA: U.S. Department of Agriculture, Natural Resources Conservation Service, Pullman Plant Materials Center. 144 p.
Peinado, M.; Aguirre, J.L.; Delgadillo, J.; Martínez-Parras, J.M. 2005. A phytosociological survey of the chionophilous communities of western North America. Part I: temperate and Mediterranean Associations. Plant Ecology. 180(2): 187-241.
Pendleton, R.L.; Pendleton, B.K. 2014. Germination patterns of a suite of semiarid grassland forbs from central New Mexico. Native Plants Journal. 15(1): 17-28.
Poreda, S.F.; Wullstein, L.H. 1994. Vegetation recovery following fire in an oakbrush vegetation mosaic. Great Basin Naturalist. 54(4): 380-383.
Poudel, A.; Satyal, P.; Swor, K.; Setzer, W.N. 2023. Essential oils of two Great Basin composites: Chaenactis douglasii and Dieteria canescens from southwestern Idaho. Journal of Essential Oil and Plant Composition. 1(3): 275-283.
Prasser, N.P.; Hild, A.L. 2016. Competitive interactions between an exotic annual, Halogeton glomeratus, and 10 North American native species. Native Plants Journal. 17(3): 244-254.
Pyle, W.H. 1993. Response of brood-rearing habitat of sage grouse to prescribed burning in Oregon. Corvallis, OR: Oregon State University. Thesis. 56 p.
Reveal J.L., Moulton G.E., Schuyler A.E. 1999. The Lewis and Clark collections of vascular plants: names, types, and comments. Proceedings of the Academy of Natural Sciences of Philadelphia. 149: 1-64.
Richardson, B.; Kilkenny, F.; St. Clair, B.; Stevenson-Molnar, N. 2020. Climate Smart Restoration Tool. https://climaterestorationtool.org/csrt/
Robinson, G.S.; Ackery, P.R.; Kitching, I.; Beccaloni, G.W.; Hernández, L.M. 2023. HOSTS: A Database of the World’s Lepidopteran Hostplants [Data set]. Natural History Museum. https://doi.org/10.5519/havt50xw
Royal Botanic Gardens Kew [RBG Kew]. 2017. Seed Information Database (SID). Version 7.1. http://data.kew.org/sid/
SEINet – Regional Networks of North American Herbaria Steering Committee [SEINet]. 2017. SEINet Regional Networks of North American Herbaria. https://symbiota.org/seinet
Shaw, N.; Pellant, M.; Fisk, M.; Denney, E. 2012. A collaborative program to provide native plant materials for the Great Basin. Rangelands. 34(4): 11-16.
Shock, C.C.; Feibert, E.B.G.; Rivera, A.; Sanders, L.D.; Kilkenny, F.; Shaw, N. 2017a. Direct surface seeding systems for the establishment of native plants in 2016. In: Shock, C.C., ed. Malheur Experiment Station Annual Report 2016. OSU AES Ext/CRS 157. Corvallis, OR: Oregon State University: 123-130.
Shock, C.C.; Feibert, E.B.G.; Rivera, A.; Saunders, L.D. 2017b. Irrigation requirements for seed production of several native wildflower species planted in the fall of 2012. In: Shock, C.C., ed. Malheur Experiment Station Annual Report 2016. OSU AES Ext/CrS157. Corvallis, OR: Oregon State University: 131-140.
Shock, C.C.; Feibert, E.B.G.; Rivera, A.; Wieland, K.; Shaw, N.; Kilkenny, F. 2019. Native wildflower seed yield in response to modest irrigation. In: Shock, C.C., ed. Malheur Experiment Station Annual Report 2018. OSU AES Ext/CrS161. Corvallis, OR: Oregon State University: 134-145.
Shock, C.C.; Feibert, E.B.G.; Saunders, L.D. 2014a. Direct surface seeding strategies for successful establishment of native wildflowers. In: Shock, C.C., ed. Malheur Experiment Station Annual Report 2013. OSU AES Ext/CrS149. Corvallis, OR: Oregon State University: 159-165.
Shock, C.C.; Feibert, E.B.G.; Saunders, L.D.; Shaw, N. 2014b. Tolerance of native wildflower seedlings to preemergence and postemergence herbicides. In: Shock, C.C., ed. Malheur Experiment Station Annual Report 2013. OSU AES Ext/CrS149. Corvallis, OR: Oregon State University: 98-206.
Sieg, C.H.; Uresk, D.W.; Hansen, R.M. 1983. Plant-soil relationships on bentonite mine spoils and sagebrush-grassland in the northern High Plains. Journal of Range Management. 36(3): 289-294.
Simonson, D.B.; Tilley, D.J. 2016. A low-cost modification to a Flail-Vac Harvester for collecting lightweight, wind-dispersed seed. Native Plants Journal. 17(2): 103-108.
Skinner, M.P. 1928. The elk situation. Journal of Mammalogy. 9(4): 309-317.
Society for Ecological Restoration; International Network for Seed Based Restoration; Royal Botanic Gardens [SER, INSR, RBGK]. 2023. Seed Information Database (SID). Available from: https://ser-sid.org/
Springer, J.D.; Stoddard, M.T.; Huffman, D.W.; Laughlin, D.C.; Fule, P.Z.; Daniels, M.L. 2022. Long-term plant community responses to resource objective wildfires in montane coniferous forests of Grand Canyon National Park, USA. Forest Ecology and Management. 515: 120224.
Stephens, G.J.; Johnston, D.B.; Jonas, J.L.; Paschke, M.W. 2016. Understory responses to mechanical treatment of pinyon-juniper in northwestern Colorado. Rangeland Ecology and Management. 69(5): 351-359.
Stevens, R.D.; Tello, J.S. 2009. Micro- and macrohabitat associations in Mojave Desert rodent communities. Journal of Mammalogy. 90(2): 388-403.
Strother, J.L. 1972. Chromosome studies in western North American Compositae. American Journal of Botany. 59(3): 242-247.
Thompson, R.S.; Anderson, K.H. 2000. Biomes of western North America at 18,000, 6000 and 0 14C yr BP reconstructed from pollen and packrat midden data. Journal of Biogeography. 27(3): 555-584.
Tilley, D. 2011. Propagation protocol for production of propagules (seeds, cuttings, poles, etc.) Machaeranthera canescens (Pursh) A. Gray seeds. Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://NativePlantNetwork.org [Accessed 2017 July 21].
Tilley, D. 2014. Release brochure for Amethyst Germplasm hoary tansyaster (Machaeranthera canescens). Aberdeen, ID: U.S. Department of Agriculture, Natural Resources Conservation Service, Aberdeen Plant Materials Center. 2 p.
Tilley, D.; Bair, C. 2011. The Jet Harvester: A shop tool for harvesting forb and shrub seeds. Native Plants Journal. 12(2): 123-127.
Tilley, D.; Ogle, D.; St. John, L. 2014. Hoary tansyaster (Machaeranthera canescens) Plant Guide. Aberdeen, ID: U.S. Department of Agriculture, Natural Resources Conservation Service, Idaho Plant Materials Center. 4 p.
Tilley, D.; Spencer, J.; Cracroft, T.; Brazee, B.; Vaughan, M.; Cruz, J.K. 2019. Creation and management of Utah butterfly habitat. Tech. Note Plant Materials 73. Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 20 p.
Tilley, D.; Tilley, N.; Fund, A.; Wolf, M. 2020. Seedling growth and competition of a late-seral, native perennial grass and 2 early-seral, native forbs in the presence of 2 densities of the invasive annual grass Bromus tectorum L. (Poaceae). Native Plants Journal. 21(3): 299-311.
Tilley, D.J. 2015a. Notice of release of Amethyst Germplasm hoary tansyaster: Selected class of natural germplasm. Native Plants Journal. 16(1): 54-60.
Tilley, D.J. 2015b. Seed production and field establishment of hoary tansyaster (Machaeranthera canescens). Native Plants Journal. 16(1): 61-66.
Tolstead, W.L. 1942. Vegetation of the northern part of Cherry County, Nebraska. Ecological Monographs. 12(3): 255-292.
Train, P.; Henrichs, J.R.; Archer, W.A. 1941. Medicinal uses of plants by Indian tribes of Nevada: Part 1. Contributions toward a flora of Nevada. No. 33. Washington, DC: U.S. Department of Agriculture, Bureau of Plant Industry, Division of Plant Exploration and Introduction. 199 p.
Turner, N.J.; Bouchard, R.; Kennedy, D.I. 1980. Ethnobotany of the Okanagan-Colville Indians of British Columbia and Washington. Victoria, BC: British Columbia Provincial Museum. 179 p.
USDA Forest Service, Bend Seed Extractory [USDA FS BSE]. 2017. Nursery Management Information System Version 4.1.11. Local Source Report 34-Source Received. Bend, OR: U.S. Department of Agriculture, Forest Service, Bend Seed Extractory.
USDA Forest Service, Bend Seed Extractory [USDA FS BSE]. 2023. Nursery Management Information System Version 4.1.11. Local Source Report 34-Source Received. Bend, OR: U.S. Department of Agriculture, Forest Service Bend Seed Extractory.
USDA Forest Service, Great Basin Native Plant Project [USFS GBNPP]. 2017. Seed weight table calculations made in-house. Report on file. Boise, ID: U.S. Department of Agriculture, Forest Service, Boise Aquatic Sciences Laboratory.
USDA Forest Service, Western Wildland Environmental Threat Assessment Center [USFS WWETAC]. 2024. TRM Seed Zone Applications. Prineville, OR: U.S. Department of Agriculture, Forest Service, Western Wildland Environmental Threat Assessment Center. Available: https://www.fs.usda.gov/wwetac/threat-map/TRMSeedZoneMapper.php
USDA Natural Resources Conservation Service [USDA NRCS]. 2024. The PLANTS Database. Greensboro, NC: U.S. Department of Agriculture, Natural Resources Conservation Service, National Plant Data Team. https://plants.usda.gov/home
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2017. Seeds of Success collection data. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program.
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2023. Bureau of Land Management technical protocol for the collection, study, and conservation of seeds from native plant species for Seeds of Success. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program. 45 p.
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2024. Seeds of Success collection data. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program.
USDI Environmental Protection Agency [USDI EPA]. 2017. Ecoregions. Washington, DC: U.S. Department of the Interior, Environmental Protection Agency. https://www.epa.gov/eco-research/ecoregions
USDI Geological Survey [USDI USGS]. 2017. Biodiversity Information Serving Our Nation (BISON). U.S. Geological Survey. https://bison.usgs.gov/#home
Utah Crop Improvement Association [UCIA]. 2015. How to be a seed connoisseur. Logan, UT: UCIA, Utah Department of Agriculture and Food, Utah State University and Utah State Seed Laboratory. 16 p.
Wahlberg, N. 2001. The phylogenetics and biochemistry of host-plant specialization in Melitaeine butterflies (Lepidoptera: Nymphalidae). Evolution. 55(3): 522-537.
Welsh, S.L. 2015. Compositae Giseke Sunflower family. In: Welsh, S.L.; Atwood, N.D.; Goodrich, S.; Higgins, L.C., eds. and contributors. Fifth Ed. Provo, UT: Brigham Young University: 137-273.
Whiting, A.F. 1939. Ethnobotany of the Hopi. Museum of Northern Arizona Bulletin No. 15. Flagstaff, AZ: Museum of Northern Arizona. 128 p.
Wolters, G.L. 1994. Elk effects on Bandelier National Monument meadows and grasslands. In: Allen, C.D., ed. Fire effects in southwestern forests: Proceedings of the Second La Mesa Fire Symposium; 1994 March 29-31; Los Alamos, NM. Gen. Tech. Rep. RM-GTR-286. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 196-205.
Yake, S.; Brotherson, J.D. 1979. Differentiation of serviceberry habitats in the Wasatch Mountains of Utah. Journal of Range Management. 32(5): 379-383.
Young, S.A.; Schrumpf, B.; Bouck, M.; Moore, M. 2020. How the Association of Official Seed Certifying Agencies (AOSCA) tracks wildland sourced seed and other plant propagating materials. Logan, UT: Utah State University, Utah Agricultural Experiment Station. 36 p.
Zeldin, J.; Lichtenberger, T.M.; Foxx, A.J.; Webb Williams, E.; Kramer, A.T. 2020. Intraspecific functional trait structure of restoration-relevant species: Implications for restoration seed sourcing. Journal of Applied Ecology. 57: 864-874.
How to Cite
Gucker, Corey L.; Shaw, Nancy L. 2018. Hoary tansyaster (Dieteria [Machaeranthera] canescens). In: Gucker, C.L.; Shaw, N.L., eds. Western forbs: Biology, ecology, and use in restoration. Reno, NV: Great Basin Fire Science Exchange. 17 p. Online: https://westernforbs.org/species/dieteria-machaeranthera-canescens-hoary-tansyaster/