Authorship
Gucker, Corey; Shaw, Nancy
Publication Date
November 2022
Nomenclature
American vetch (Vicia americana Muhl. ex Willd.) belongs to the Fabaceae (formerly Leguminosae) family, Vicieae tribe (Endo and Ohashi 1997), Americanae section, and Cracca subgenus (Endo et al. 2008).
Family
Fabaceae – Pea family
Genus
Vicia
Species
americana
NRCS Plant Code
VIAM (USDA NRCS 2021).
Subtaxa
There are two American vetch subspecies currently recognized in North America: V. a. subsp. americana and V. a. subsp. minor (USDA NRCS 2021).
Synonyms
The following proposed varieties: americana Muhl. ex Willd., angustifolia Nees, linearis (Nutt.) S. Watson, minor Hook., oregana (Nutt.) A. Nelson, truncata (Nutt.) W.H. Brewer, villosa (Kellogg) F.J. Herm., and subspecies: oregana (Nutt.) Abrams are considered synonyms (ITIS 2022).
Common Names
American vetch, peavine, wild pea, and wild vetch (Craighead et al. 1963; Blackwell 2006; Courtemanch and Kauffman 2008).
Chromosome Number
2n = 14 (Hickman 1993; Welsh et al. 2016).
Hybridization
In the reviewed literature, hybridization was not reported.
Distribution
American vetch grows throughout North America. In the United States, it grows in all but the southeastern states, and it is widely distributed west of the Mississippi River (Hickman 1993; Allen and Tilley 2014). It is widespread in the Intermountain Region but becomes less common toward the arid southwestern edge (Barneby 1989). Variety americana occupies the range described for the species (Allen and Tilley 2014; Hitchcock and Cronquist 2018). Variety minor is most common in short-grass prairies and dry woodlands east of the Rocky Mountains, but also occurs in western Montana and eastern Idaho (Allen and Tilley 2014; Hitchcock and Cronquist 2018).
Habitat And Plant Associations
As its broad distribution suggests, American vetch grows in a variety of habitats from arid (10 in [250 mm] annual ppt) to mesic (≤ 50 in [1,270 mm] annual ppt) (Allen and Tilley 2014). It grows from valley and foothill grasslands, shrublands, and woodlands to high-elevation meadows and alpine forests (Hermann 1966; Barneby 1989; Welsh et al. 2016). American vetch is often associated with disturbed sites such as roadsides (Fig. 1), fencerows, old fields, canal banks, and stream banks (Hickman 1993; Spellenberg 2001; Welsh et al. 2016).
In grasslands, open woodlands, and thickets in prairie habitats, American vetch was common on north slopes and associated with common yarrow (Achillea millefolium) and northern bedstraw (Galium boreale) (Currah et al. 1983). In Montana’s Missouri River Breaks, American vetch occurred in most grasslands (Agropyron and Poa spp.), shrublands (big sagebrush [Artemisia tridentata], greasewood [Sarcobatus vermiculatus], rough cocklebur [Xanthium strumarium]), woodlands (Juniperus scopulorum), and forests (ponderosa pine [Pinus ponderosa], Douglas-fir [Pseudotsuga menziesii]) (Mackie 1970). In an analysis of vegetation patterns in the Crested Butte area of west-central Colorado, American vetch occurrence and cover were greater in quaking aspen (Populus tremuloides) woodlands (100%, 6.6%) than in big sagebrush shrublands (85%, 0.9%) or Thurber’s fescue (Festuca thurberi) grasslands (79%, 1.1%) (Langenheim 1962).

Figure 1. American vetch growing along a two-track dirt road in a big sagebush-grassland community. Photo: M. Lavin, Montana State University (MSU).
Grasslands. American vetch is reported in various grassland types from western Canada to northern Nebraska. In descriptions of rangeland cover types of the US, it is common in the wheatgrass-grama, wheatgrass, and fescue rangeland cover types (Shiflet 1994). The wheatgrass-grama grassland is dominated by western wheatgrass (Pascopyrum smithii), green needlegrass (Nassella viridula), and grama species (Bouteloua spp.). This grassland type occurs west of the Missouri River from Montana and South Dakota to the extreme northwestern part of Nebraska where average annual precipitation ranges from 5 to 6 in (130–150 mm), and there are typically 130 to 150 frost-free days each year. The wheatgrass cover type is primarily found in northwestern and central South Dakota and is dominated by wheatgrasses and green needlegrass. The fescue cover type, also referred to as the submontane mixed prairie or foothill prairie type, is dominated by plains rough fescue (F. hallii) and found around aspen parklands and the boreal forest zone in Alberta, Saskatchewan, the Rocky Mountains of southern Montana, and scattered locations in northern North Dakota. The fescue cover type occurs where average annual precipitation ranges from 12 to 19 in (305–483 mm), and the frost-free period is 80 to 120 days (Shiflet 1994).
In western Canada, American vetch was described as constant in Altai fescue-Richardson’s needlegrass (F. altaica–Achnatherum richardsonii) grasslands (Looman 1969). In an evaluation of temporal changes in species composition of fescue (plains rough fescue and rough fescue [F. campestris]) grasslands at the University of Saskatchewan’s Kernan Prairie, American vetch abundance decreases were associated with increases in cold-stress days from March to April. Cold-stress days were determined as the cumulative average daily temperature after 5 consecutive days where the average daily temperature was less than 32 °F (0 °C) (Gross and Romo 2010).
In the western U.S., American vetch occurs in grasslands throughout its range (Fig. 2). In a study of Deer Park in Olympic National Park by del Moral (1984), American vetch occurred in dry Idaho fescue (F. idahoensis) meadows but not in wet showy sedge (Carex spectabilis) meadows. In western Montana, American vetch occurred in bluebunch wheatgrass (Pseudoroegneria spicata), western wheatgrass, and fescue grassland types, and its constancy was typically greatest in the bluebunch wheatgrass type (Mueggler and Stewart 1980). In late seral Idaho fescue-bluebunch wheatgrass grasslands near Bozeman, Montana, frequency of American vetch was 88 to 97% (LeFebvre et al. 2017). In western North Dakota, American vetch occurred in late-seral grasslands dominated by western wheatgrass and thickspike wheatgrass (Elymus lanceolatus) growing where annual precipitation averaged 15 in (380 mm), and the average date of the first killing frost was May 20 and the last was September 15 (Quinnild and Cosby 1958).
 Figure 2. American vetch growing in a dry grassland habitat in Nevada. Photo: USDI, Bureau of Land Management (BLM) NV-30 Seeds of Success (SOS).
Figure 2. American vetch growing in a dry grassland habitat in Nevada. Photo: USDI, Bureau of Land Management (BLM) NV-30 Seeds of Success (SOS).
Shrublands. American vetch is a principal forb in the sagebrush-grassland rangeland cover type. This cover type occurs in the northern Great Plains and is dominated by big sagebrush and often includes silver sagebrush (A. cana), rabbitbrushes (Chrysothamnus viscidiflorus and Ericameria nauseosa), and bluebunch wheatgrass. The type occurs at elevations of 2,600 to 4,500 ft (790–1,400 m) where annual precipitation averages 11 to 16 in (280–400 mm) with most falling in spring and early summer (Shiflet 1994). In the dry zone of subalpine meadow vegetation near Gunnison, Colorado, American vetch commonly grows with big sagebrush, Thurber’s fescue, oblongleaf bluebells (Mertensia oblongifolia), and fivenerve helianthella (Helianthella quinquenervis) (Harte and Shaw 1995).
Forests/woodlands. American vetch is associated with various woodland and forest types in North America which are presented from northern to southern locations below. An Oregon white oak (Quercus garryana)/Idaho fescue-American vetch plant community was described at the northern margin of Oregon white oak habitats in British Columbia (Erickson 2002). In the boreal mixed-wood near Lac La Biche, Alberta, American vetch was an indicator species for broadleaf-dominated communities when understory vegetation was compared for four canopy types: conifer (white spruce [Picea glauca])-dominated forests, broadleaf (quaking aspen)-dominated woodlands, mixed conifer-broadleaf forests, and canopy gaps (Chávez and Macdonald 2010).
A Douglas-fir-Pacific madrone (Arbutus menziesii)/American vetch community type was described on south-facing slopes of Sucia Island in Puget Sound, Washington. In this community, where winds were moderate, temperatures high, and soil moisture low, cover of American vetch averaged 16.2%. American vetch also occurred, but with much lower cover (2.8%) in fescue grasslands on the island in areas where the growing season was hot, dry, and windy (Fonda and Bernardi 1976).
In a study of forests in the south Umpqua drainage of the Umpqua National Forest, constancy of American vetch was 91% in warm dry, 55% in cool moist, and 46% in cold moist mixed-conifer forests. It was considered a major understory species in Shasta red fir (Abies magnifica var. shastensis)-dominated forests (Franklin and Dyrness 1973).
Along the northern Missouri River bottom lands, American vetch was common in plains cottonwood (Populus deltoides subsp. monilifera) stands in north-central Montana (Walcheck 1970) and eastern cottonwood (P. deltoides) stands in west-central North Dakota (Johnson et al. 1976). The floodplain in North Dakota had not experienced significant flooding in the last 20 years (Johnson et al. 1976).
In northern California, American vetch occurs in a variety of woodland and forest types. On volcanic soils in the South Warner Mountains, American vetch occurred in 33 to 65% of the quaking aspen, white fir (A. concolor), Jeffrey pine (Pinus jeffreyi), and ponderosa pine stands where overstory canopy cover ranged from 10 to 75% (Riegel et al. 1990). In the northern Sierra Nevada Mountains of Plumas County, California, American vetch grew in the depauperate understory of late-seral Jeffrey pine forests with some incense cedar (Calocedrus decurrens) and white fir. Jeffrey pine trees averaged 35 in (88 cm) in diameter at breast height (DBH) and were 100 to 226 years old (Riegel et al. 2002). In the Redwood Creek basin of Redwoods National Park, American vetch was an important associate of the Oregon white oak/orchardgrass (Dactylis glomerata) woodland type. Although most abundant in this woodland type, it occurred in all Oregon white oak communities and in open glades and dense shrublands along streams (Sugihara et al. 1987).
In the Intermountain Region, American vetch was described in Gambel oak stands in central and northern Utah (Kunzler et al. 1981) and in quaking aspen stands in Gunnison County, Colorado (Morgan 1969). In Utah, frequency of American vetch was up to 12%. When Gambel oak was replaced by bigtooth maple (Acer grandidentatum), American vetch was no longer in the understory (Kunzler et al. 1981). Occurrence of American vetch was 100%, and it grew as thick entangled mats in 25 quaking aspen stands in Gunnison County, Colorado. Quaking aspen stands in this area grew as islands supporting primarily 4- to 7-in (10–18 cm) DBH sized trees and were surrounded by fescue grasslands (Morgan 1969).
American vetch occurs in a variety of forests in the southwestern US. It often occurs in the understory of white fir, Douglas-fir, ponderosa pine, twoneedle pinyon (P. edulis) forest types growing in Utah, southern Colorado, Arizona, and New Mexico (Larson and Moir 1987). In the northern part of the Cibola National Forest of New Mexico, American vetch was common in the understory of forests dominated by Engelmann spruce (Picea engelmannii), subalpine fir (Abies lasiocarpa), blue spruce (Picea pungens), white fir, and Douglas-fir. Cover of American vetch was often highest (but ≤ 20%) in the early-seral forest stages dominated by quaking aspen (Alexander et al. 1987).
Elevation
The elevation range reported for American vetch in the western U.S. is 3,600 to 11,700 ft (1,100–3,570 m) (Andersen and Holmgren 1996; Blackwell 2006). In the Intermountain West, it grows at 3,600 to 9,500 ft (1,100–2,900 m) elevations (Barneby 1989). The elevation range reported for American vetch in California is below 7,900 ft (2,400 m) (Hickman 1993) and in Utah is 4,200 to 11,700 ft (1,270–3,570 m) (Welsh et al. 2016).
Soils
American vetch grows in fine to coarse-textured soils. It tolerates slightly saline, moderately acidic, and dry soil conditions. In western mountain habitats, it is often abundant in deep porous loams rich in organic matter. In plains and foothill habitats, it is common in clayey soils (Wasser and Shoemaker 1982; Currah et al. 1983; Barneby 1989; Allen and Tilley 2014).
American vetch characterized loamy sands when areas with and without western harvester ant (Pogonomyrmex occidentalis) mounds were compared in semi-arid ponderosa pine communities near Los Alamos, New Mexico. Cover of American vetch was significantly greater away from mounds (P < 0.05) where soil nutrients and water availability were lowest (Carlson and Whitford 1991).
Soil depth. In some habitats, American vetch is associated with shallow soils. At the northern margin of Oregon white oak habitats in British Columbia, the Oregon white oak/Idaho fescue-American vetch community was associated with shallower soils than other Oregon white oak communities (Erickson 2002). In Montana, American vetch was also considered a dominant species in climax western wheatgrass, thickspike wheatgrass, and bluebunch wheatgrass grassland types on clay soils that were sometimes shallow (Ross and Hunter 1976).
Soil moisture. American vetch often occupies dry soils, but the species also grows on floodplains with deep water tables. In southern boreal forests in Saskatchewan and Manitoba, American vetch was indicative of the fourth driest soil type when five soil moisture types from very dry to wet were compared (Rowe 1956). Near Gunnison, Colorado, American vetch was common in the dry subalpine meadow zone where soils and snowmelt were evaluated. At 2 to 10 in (5–25 cm) depths, average soil moisture ranged from 17 to 28%, snow melt date (Julian) ranged from 118 to 145, and soil temperature ranged from 55 to 59 °F (13–15 °C) for the 1991–94 study period (Harte and Shaw 1995). On the Middle Loup River floodplain in central Nebraska, American vetch occurred only where the water table was 40 in (101 cm) or deeper (Nagel 1995).
Soil texture and nutrients. In descriptions of rangeland cover types of the U.S., American vetch is common in the wheatgrass-grama, wheatgrass, and sagebrush-grassland rangeland cover types. The wheatgrass-grama type occurs on heavy clay to silty shale soils. The wheatgrass cover type occurs on various soils including dense clays. The sagebrush-grassland rangeland cover type occupies variable soils but is most frequent on silts and clays (Shiflet 1994). In central Montana, American vetch is common in mountain big sagebrush (Artemisia tridentata subsp. vaseyana) grassland types on gentle to steep slopes in loams or clays (Smith 1969).
In an analysis of the environmental variables most strongly correlated with the composition of ponderosa pine understories near Flagstaff, Arizona, American vetch was commonly associated with loam and silt loam soils. These soil types occurred on limestone and moist basalt sites and had pH levels ranging from 5.7 to 6.4 (Abella and Covington 2006).
American vetch occurred on serpentine-like soils in California but not in Washington. In a study conducted along the upper North Fork of the Teanaway River in Washington’s Wenatchee Mountains, American vetch occurred in the openings of forests growing on sandstone but not on serpentine soils (del Moral 1982). In the Pine Hill region of El Dorado, California, American vetch occurred on gabbro soils, which were like serpentine soils but with higher calcium to magnesium ratios and lower concentrations of nickel and chromium (Wilson et al. 2009).
Description
American vetch is a highly variable perennial with a deep taproot (Barneby 1989; Hitchcock and Cronquist 2018). Plants are slender with weak stems up to 50 in (127 cm) long, leading to a clambering (Fig. 3) or reclining growth form (Barneby 1989; Andersen and Holmgren 1996; Welsh et al. 2016; Luna et al. 2018). Plants are multi-branched with square stems (Craighead et al. 1963) and variable in size, form, pubescence, and leaf size and shape (Barneby 1989; Welsh et al. 2016).

Figure 3. American vetch climbing on associated vegetation. Photo: M. Lavin, MSU.
American vetch produces a moderately deep (<40 in [100 cm]) branched taproot (Allen and Tilley 2014) with rhizomes and substantial fibrous roots (Currah et al. 1983; Pokorny et al. 2004). The root system of a single plant having five separate above-ground stems was described after excavation from the Boise River watershed in Idaho (Woolley 1936). The root system had extensive laterals in the top 8 in (20 cm) of soil that produced the above-ground stems and a network of many fibrous roots. Root spread was a little more than 24 in (60 cm), and roots reached a little more than 40 in (100 cm) deep (Woolley 1936). Root growth and development were rapid following seeding of American vetch on a deep, silty clay loam creek bottom soil near Manhattan, Kansas. Root size was also much large than that reported above, suggesting that roots may grow much larger without competition in loose, deep soils (Gibbens 1954, see the Seed and Seedling Ecology section below for details).
Plants are extremely variable in the thickness, pubescence, and size of leaves (Gould et al. 2013; Welsh et al. 2016). Leaves and stems are hairless to pubescent (Welsh et al. 2016; Luna et al. 2018). Alternate leaves are pinnately compound with 6 to 18 leaflets and a terminal leaflet modified into a branched or unbranched tendril (Hermann 1966; Allen and Tilley 2014; Luna et al. 2018). Leaflet peduncles are shorter than the leaflets (Munz and Keck 1973). Leaflets can be wedge-shaped or oval to linear with rounded to sharply pointed tips (Hickman 1993; Luna et al. 2018). Leaflets are strongly veined with simple to forked or branched terminal tendrils (Fig. 4) (Currah et al. 1983; Gould et al. 2013).

Figure 4. American vetch’s compound leaves, individual leaflets, and terminal tendrils. Photo: M. Lavin, MSU.
Inflorescences are loose racemes measuring up to 10 in (25 cm) long (Kenicer and Norton 2008; Welsh et al. 2016) that bear 2 to 10 rose to purple flowers that transition to blue with age (Fig. 5) (Hickman 1993; Andersen and Holmgren 1996; Spellenberg 2001; Welsh et al. 2016; Luna et al. 2018). Papilionaceous flowers are produced on peduncles (1–3 in [3–8 cm]) and are usually borne on just one side of the axis. Flowers consist of a five-toothed campanulate calyx (6.2–8.4 mm long), tube (4.8–6.5 mm long), and body (23–35 mm long and 6–8 mm wide). The reflexed banner is longer than the keel, and flowers open more widely at anthesis (Barneby 1989; Hickman 1993; Kenicer and Norton 2008; Welsh et al. 2016; Luna et al. 2018). Flower tubes are 10-nerved to the middle then five-nerved apically (Barneby 1989). Flowers produce one free stamen and a group of nine stamens with fuse filaments that surround the ovary (USDA FS 1937).

Figure 5. American vetch inflorescences. Note the change in color from pink to blue as flowers age. Photo: M. Lavin, MSU.
American vetch produces two to five seeds in dry, narrowly elliptic pods measuring 0.8 to 1 in (2–3 cm) long and 0.5 to 1 cm wide (Fig. 6) (Hickman 1993; Hitchcock and Cronquist 2018; Luna et al. 2018). Pods are mostly glabrous and coil upon dehiscence (Hickman 1993; Gould et al. 2013). Seeds are 4 to 5 mm in diameter, subspherical, spherical, or oblong, smooth, and dull purplish brown to dull light brown with scattered to dense mottles. Some seed is so densely speckled it appears to be a single color (Gunn 1970; Munz and Keck 1973; Gould et al. 2013).

Figure 6. American vetch pods and immature seeds. Photo: BLM NV030, SOS.
Varieties. American vetch varieties can be distinguished by the number of flowers per inflorescence and growth form (Allen and Tilley 2014). Variety americana plants are larger and more sprawling with trailing or climbing stems reaching 12 to 40 in (30–100 cm) long (Barneby 1989; Hitchcock and Cronquist 2018). Variety minor stems are mostly erect and less than 40 in (100 cm) long. Tendrils of variety americana are generally forked and prehensile, while those of minor are not or scarcely prehensile and occasionally reduced to short bristles. Variety americana leaflets are highly variable in shape and racemes have three to nine flowers. Variety minor leaflets are narrowly oblong to linear, and racemes have two to five flowers (Barneby 1989; Hitchcock and Cronquist 2018). Varieties can also occupy somewhat different ranges (see the Distribution section).
Lookalikes. American vetch and other vetch (Vicia) species closely resemble and can be confused with pea (Lathyrus) species (Welsh et al. 2016). The two genera can be distinguished by leaflet venation, corolla fusion, and hairs on the styles (Hermann 1966; Spellenberg 2001). Lateral veins of leaflets are oriented about 60 degrees from the midvein for vetch species and about 30 degrees for pea species. Wing petals are united with the keel of the corolla for vetch species and free or nearly so for pea species (Hermann 1966). Hairs surround the tip of the style like a shaving brush in vetch species. Hairs occur along the inner face of the style in pea species (USDA FS 1937; Hermann 1966; Spellenberg 2001). For more on distinguishing characteristics, see Kenicer and Norton (2008).
Belowground Relationships And Interactions
American vetch produces root nodules that contain nitrogen-fixing bacteria (USDA FS 1937). A review by Gould et al. (2013) reports that American vetch roots were associated with vesicular arbuscular mycorrhizal fungi (AMF) and rhizobial bacteria, but the species were not identified.
In a controlled study, growth of American vetch was not significantly different when seed was grown in soil with or without AMF. The AMF used in the study was extracted from field soil collected in Custer County, Montana, or from the roots of plants growing in same location (Reinhart et al. 2017).
Reproduction
American vetch reproduces from seed and rhizomes (Currah et al. 1983; Wiens 1984). Seed production is reduced in dry conditions (Weaver et al. 1935). Analysis of 10 American vetch plants growing in Utah’s Wasatch Mountains revealed that 37% of ovules developed into seeds (Wiens 1984). An average of 62 seeds was collected from single stems of average-size plants growing in North Dakota (Stevens 1957). Plants can be propagated from rhizome cuttings (Currah et al. 1983). Plants produced few flowers in a tallgrass prairie near Lincoln, Nebraska, during the great drought of 1934 (Weaver et al. 1935).
Flowering and seed production were low for plants growing along a south-facing section of the Attawapiskat River floodplain in north-central Canada. Number of ramets, flowers/ramet, and flowers/plot were evaluated on untreated and fertilized (4 g/m2 of N, P, and K) plots for two years. American vetch produced flowers and seed on just one of 10 plots, but ramet production increased with fertilization (P = 0.025) (Rantala-Sykes and Campbell 2018).
Phenology
American vetch typically emerges in early spring, flowers in June or July, produces ripe seed in July or August, and dies back in late August or September (Currah et al. 1983). Vegetative growth or regrowth continues almost all summer with available moisture at higher elevations, but plants go semi-dormant with dry conditions and high temperatures at lower elevations (Wasser and Shoemaker 1982). Flowering date can vary from April to August and is affected by location, elevation, and local weather (Munz and Keck 1973; Barneby 1989; Ogle et al. 2017). In Alberta, ripe seeds could be produced from July through September (Gould et al. 2013).
First flowering was earlier in warm years than in cool years during observations made in mixed-grass prairie near Woodworth, North Dakota. Flowering was monitored from 1979 to 1984. The earliest first bloom was May 23, 1984, and the latest first bloom was June 6, 1979. The median date of full flowering was June 4, and the median date at which flowering was 95% complete was July 1 (Callow et al. 1992).
American vetch flowering has begun earlier as spring temperatures have increased and growing seasons have lengthened over time. In the Northern Great Plains, the first flowering date was significantly earlier in contemporary (2007–2010) time than historic time (1940–1961) (P < 0.05), which corresponded with increased spring temperatures and longer growing seasons in the area (Dunnell and Travers 2011). Phenology data gathered from 1974 to 2012 in a subalpine habitat in Colorado’s Rocky Mountains revealed that American vetch plants showed a significant increase in peak flower abundance over time (P = 0.018) (CaraDonna 2016).
Pollination
Seed production requires insect pollination. Seeds mature about 1 month after flowers are pollinated (Allen and Tilley 2014). Large-bodied bees including bumble bees (Bombus spp.) are the most common pollinators (Harder and Cruzan 1990; Forrest et al. 2010).
In a study of flower size and bee visitation in a subalpine meadow location at the Rocky Mountain Biological Laboratory (RMBL), Colorado, the corolla tube of American vetch flowers averaged 0.5 in (12.4 mm) long, which was long for the native flowers evaluated. Flowers were visited by white-shouldered bumble bee (B. appositus) queens and workers and yellow-fronted bumble bee (B. flavifrons) workers. Time spent by bumblebees per flower was significantly correlated with proboscis length (P < 0.05) (Inouye 1980). Flowers were also visited by leaf-cutter bees (Megachile spp.), mason bees (Osmia spp.), and bumble bees, which were thought to be important pollinators because of their large size (Forrest et al. 2010). In the same location, American vetch flowers were also visited by Apidae, Halictidae, and Megachilidae bees and Bombyliidae and Syrphidae flies in at least one observation year. Visitation was observed for 1 hour each week on 24 plots between 9 am and 4 pm from June 2005 through August 2007. This study also evaluated nutrient enrichment, which did not affect plant-pollinator networks (Burkle and Irwin 2009). American vetch flowers on plants growing near Calgary, Alberta, were primarily pollinated by bumble bees and large leaf-cutter bees (Harder and Cruzan 1990).
Ecology
American vetch is an early colonizer and common on disturbed sites (Shantz 1906) but also grows in late-seral and climax vegetation (Ross and Hunter 1976; Larson and Moir 1987). Plants have been described as long-lived (Gould et al. 2013), but life span can vary by location and was considered short-lived in humid regions (Wasser and Shoemaker 1982). In a study of grama grasslands on mesas east of Pikes Peak, Colorado, American vetch occurred on any site where vegetation cover was removed by heavy grazing, road creation, or flooding (Shantz 1906). It occurred on roadside cuts and in late-seral vegetation in fescue grasslands, Douglas-fir forests, and quaking aspen woodlands in the Northern Rocky Mountains of Montana and Wyoming (Weaver et al. 1993).
American vetch is shade tolerant, but its abundance is often greater in full sun or partial shade than full shade. In open meadow and quaking aspen groves near Crested Butte, Colorado, the ratio of American vetch frequency in the sun to its frequency in the shade was 0.6 (Louda et al. 1987). Growth differences were not significantly different in shade and sun habitats. Plants averaged 9.5 in (24.2 cm) tall in the sun and 11.5 in (29.1 cm) tall in the shade. Leaf length averaged 18 mm in the sun and 21 mm in shade. Leaf area damage to American vetch was significantly greater for plants in the sun than in the shade (P <0.05) (Louda et al. 1987). In ponderosa pine forests in South Dakota’s Black Hills Experimental Forest, productivity of American vetch in clearcuts was more than 4 times that in unthinned stands or stands managed at 21 to 122 ft2/ac (5–28 m2/ha) basal area stocking levels in one year of understory evaluation. In another year, productivity of American vetch was greatest (19 lbs/ac [22 kg/ha]) in stands managed at 39 ft2/ac (9 m2/ha) stocking (Uresk and Severson 1998). When clearcuts, thinned, and undisturbed forests were compared in the Siskiyou Mountains of southwestern Oregon, the maximum cover of American vetch was more than 60% in full light conditions. Maximum cover was 50% at 25 to 60% full light, 25% at 11 to 25% full light, and 5% or less at less than 11% full light (Emmingham 1972).
Seed And Seedling Ecology
American vetch exhibits ballistic seed dispersal; seed is scattered widely when the dehiscent fruits split. Seedlings grow rapidly but growth and development can vary by soil composition.
Although Gould et al. (2013) reports that seed can be shot up to 16 ft (5 m) from the pod, the presence of American vetch seeds in the seed bank was often limited to areas where it currently or very recently occurred in the aboveground vegetation. In semi-desert shrublands in British Columbia’s southern Okanagan Valley, density of American vetch seed was 0.009/ft2 (0.1/m2) in the seedbank and plants were 0.5/ft2 (6/m2) in the aboveground vegetation. Three soil cores (4 in [10 cm] deep, 0.8 in [1.9 cm] diameter) were taken from 40 plots from each of 10 grazed and ungrazed sites (Clements et al. 2007). In dry Douglas-fir forests near Kamloops, British Columbia, seedlings emerged only from soil where plants also occurred in the aboveground vegetation (Stark et al. 2006).
Seedling root growth and development were rapid for American vetch seedlings growing without competition on a deep, silty clay loam floodplain near Manhattan, Kansas (Gibbens 1954). The study site was plowed to 6 in (15 cm) deep, harrowed, and rolled with a heavy packer before the early May seeding with locally collected seed. Plots were sprinkler irrigated and protected from desiccation with leaf mulch. Seedlings emerged 12 to 17 days after seeding. At 3 days old, seedling stems averaged 0.7 in (1.8 cm) long with 4 leaves and 2 leaflets, and roots extended 3.2 in (8.1 cm) deep. At 3 weeks old, seedling stems averaged 3 in (7.6 cm) tall, and roots reached 14.2 in (36 cm) deep. Stems branched at 4 weeks old. Flowers first appeared as early as 11 weeks old. At 13 weeks, plants occupied 7 × 19 in (18 × 48 cm) of aboveground space. At the end of the growing season, taproots were large in the top 12 in (30 cm) of soil, there were 12 major lateral roots, and the root system was 60 in (150 cm) wide and deep. This seedling development represents growth for plants that were watered, kept free of competition, and grown in deep alluvial soils (Gibbens 1954).
Growth of one- and two-year-old seedlings was reported in nutrient addition field experiments at North Dakota State University’s Albert Ekre Grassland Preserve (Bingham and Biondini 2009). Nitrogen and phosphorous were applied in early spring each year. American vetch was seeded with other species in fall 1998 and spring 1999. Plant growth and nutrient use efficiency were evaluated in 2000. Root to shoot ratio averaged 1.01, and the relative growth rate was 0.08 g/g·d. Nitrogen use efficiency averaged 38.17 g/g·d, and phosphorous use efficiency averaged 102.65 g/g·d (Bingham and Biondini 2009).
Greenhouse studies suggest that the roots of American vetch seedlings produce nodules readily when seeded in native soils. When emergence was compared in sterilized soils and soils containing rhizobia, emergence was much slower in the sterilized soils (Keeler and Rafferty 2018). In a study evaluating whether the presence of bryophytes affected the germination and establishment of vascular plants, American vetch root nodulation the occurred in field-collected burned soil lacking bryophytes, burned soil with bryophytes, and unburned soil with bryophytes (Kayes 2008). There were no significant differences in seedling growth or root to shoot biomass by soil type. American vetch seed was wild collected near where the soil collections were made in southwestern Oregon (Kayes 2008).
Disturbance Ecology
American vetch is highly tolerant of disturbance. It colonizes open sites when a seed source or rhizome piece is available (Fig. 7). It also typically recovers from surviving root systems following top-killing disturbances. During the great drought from 1933 through 1940, American vetch decreased almost to the point of vanishing in mid-continental grasslands. It reappeared in considerable abundance by 1942 with the return of moisture (Albertson and Weaver 1944). In a field experiment in native mixed-grass prairie in central Saskatchewan, abundance of American vetch increased six times with heavy litter additions (7.5 oz/ft2 [2,290 g/m2]). Internode elongation of established plants allowed them to penetrate the litter layer based on field observations (Letts et al. 2015).

Figure 7. American vetch colonizing an open, recently disturbed site. Photo: M. Lavin, MSU.
Grazing. American vetch is highly palatable to livestock and native grazers and sensitive to trampling (Gould et al. 2013). Because of this, it is typically more abundant on lightly grazed or ungrazed sites than heavily grazed sites.
In Riding Mountain National Park, western Canada, frequency of American vetch was greater on lightly grazed than heavily grazed plots and increased with time following removal of cattle (Sinkins and Otfinowski 2012). The relative density of American vetch was typically greater on ungrazed or moderately grazed sites compared to sites heavily grazed by cattle in the Central Grasslands Research Center located northwest of Streeter, North Dakota. The ungrazed area had not been used since 1979. Moderate grazing left about 50% of the long-term peak above-ground biomass of ungrazed sites, while heavy grazing left about 10% of the biomass of ungrazed sites (Table1; Biondini et al. 1998).
Table 1. Average relative density of American vetch on sites ungrazed, moderately grazed, and heavily grazed by cattle at the Central Grasslands Research Center, North Dakota (Biondini et al. 1998).
| Year | 1988 | 1990 | 1991 | 1992 | 1993 | 1994 | 1995 |
| Average relative density (%) (SE in parentheses) | |||||||
| Ungrazed | 0 (0.0) | 3(1.5) | 9(5.4) | 6(2.5) | 11(3.9) | 6(1.4) | 5(1.2) |
| Moderate grazing | 1(0.5) | 9(1.9) | 8(3.1) | 5(1.6) | 8(2.4) | 6(1.7) | 5(1.8) |
| Heavy grazing | 1(0.4) | 6(0.2) | 6(2.8) | 2(0.5) | 4(1.2) | 3(1.0) | 3(0.7) |
Although American vetch abundance was often greater on protected or lightly grazed sites, some studies reported the opposite. When Wyoming big sagebrush (Artemisia tridentata subsp. wyomingensis) and northern mixed-prairie vegetation were compared within and outside of long-term exclosures in Wyoming’s Thunder Basin National Grassland, American vetch was associated with areas outside of the exclosures. The exclosures protected vegetation from livestock and pronghorn (Antilocapra americana) for 53 to 80 years (Porensky et al. 2020). (See also, Wildlife and Livestock Use section).
Fire. Abundance of American vetch is often unchanged or increased following fire in grasslands, shrublands, and forests (Cook 1959; Anderson and Bailey 1979; Merrill et al. 1980; Halpern 1989). McLean (1969) characterized American vetch as moderately resistant to fire based on observations made on 1- to 19-year-old lightly to heavily burned sites and its production of fibrous roots and rhizomes at 0.6 to 2 in (1.5–5 cm) below the soil surface.
American vetch tolerated multiple years of burning in Canadian grasslands (Anderson and Bailey 1980; Gross and Romo 2010). It was an indicator species on three-time burned sites (P = 0.048) at the University of Saskatchewan Kernan Prairie. Vegetation composition was evaluated on unburned plots and plots burned one to three times in all months except January and February. Burned plots were visited 1 to 6 years after fires (Gross and Romo 2010). In fescue grasslands in east-central Alberta, American vetch frequency and cover were almost double on annually burned than on unburned plots (P < 0.0005). Burned plots were burned in April each year for at least 24 years (Anderson and Bailey 1980).
In studies that compared fire season and fire severity, American vetch was typically more sensitive to growing-season and high-severity fires, but the responses were variable and American vetch was rarely absent from burned plots regardless of fire severity or season. It occurred in the second post-fire growing season following fall but not spring or summer prescribed fires in the Altai fescue-dominated Kernan Prairie in central Saskatchewan (Archibold et al. 2003). American vetch was absent from burned plots following a mid-summer wildfire in sagebrush-grassland vegetation in central Utah. Production of American vetch was 7.75 lb/ac (8.68 kg/ha) before the fire, but it was not sampled in plots visited 1 and 2 years after fire (West and Hassan 1985).
Cover of American vetch was greater in the burn perimeter than in unburned or interior-burned plots following a rare winter (December 14, 1997) fire in a rough fescue grassland in southwestern Alberta. The fire was extremely hot and vegetation recovery was slowest in the interior of the burn (Table 2; Bork et al. 2002).
Table 2. Average cover of American vetch inside and outside of the burn perimeter 1 and 2 years following a winter fire in a grassland in southwestern Alberta. There were no species-level significance tests (Bork et al. 2002).
| Year | Unburned | Burn perimeter | Burn interior |
| ———Cover (%)——— | |||
| 1998 | 0.4 | 1.3 | 0.6 |
| 1999 | 1.3 | 2.0 | 0.1 |
The cover and frequency of American vetch were significantly greater on low-severity burned than unburned plots or high-severity burned plots following a prescribed fire in a ponderosa pine forest on the Coeur d’Alene Reservation in Benewah County, Idaho. The study area was unburned for 44 to 62 years, ungrazed by livestock for at least 30 years, and selectively logged about every 10 years before the prescribed fire (Table 3; Armour et al. 1984).
Table 3. Average cover and frequency of American vetch after prescribed burning in a ponderosa pine forest on the Coeur d’Alene Reservation in Benewah County, Idaho (Armour et al. 1984).
| Treatment | Unburned | Low-severity burn | High-severity burn |
| Average % from 1, 2, and 3 yrs after prescribed fire | |||
| Cover | 0.1b | 0.7a | 0.1b |
| Frequency | 1.2b | 11.1a | 2.1b |
Cover (2.7%) and frequency (42%) of American vetch were much greater where the forest floor was entirely consumed by fire than where litter but no duff (0.3% cover, 16% frequency) was consumed or where duff remained but almost no litter (0.1% cover, 6% frequency) was consumed. These findings were based on an average of the first 4 post-fire years after an early May fire in boreal mixed-wood stands in southeastern Manitoba (Wang and Kemball 2005).
American vetch was an important species for distinguishing unburned and high-severity burned sites 2 years after early summer fires in northern Arizona ponderosa pine forests. Average cover of American vetch was 4.9% on high-severity burned and 0.5% on unburned plots. The density of trees on high-severity burned plots was 0 to 16 live trees/ac (0–40/ha) and 45 to 469 dead trees/ac (110–1,160/ha). Unburned plots supported 32 to 368 live trees/ac (80–910/ha) and 2 to 18 dead trees/ac (4-44/ha (Crawford et al. 2001).
The production of American vetch was greater on low-severity than unburned or high-severity burned plots soon after an early May 1972 wildfire in ponderosa pine forests on the Coconino National Forest near Flagstaff, Arizona (Table 4; Bataineh et al. 2006). Production was more variable as the time since fire increased in the study that compared understory vegetation recovery on low-severity, high-severity, and unburned plots from immediately following the fire to 30 years following the fire. In the second post-fire year, American vetch was an indicator species on low-severity burned sites (P = 0.0014), but by 8 years following fire, production was eight times greater on unburned than high-severity or low-severity burned plots. The burned area was salvage logged in the first post-fire year. Skid trails were seeded with a mix of forbs and grasses that did not include American vetch in the third post-fire year. Post-fire monitoring avoided skid trails (Table 4; Bataineh et al. 2006).
Table 4. Production of American vetch on low-severity burned, high-severity burned, and unburned plots following an early May 1972 wildfire in ponderosa pine forests on the Coconino National Forest, Arizona (Bataineh et al. 2006).
| Year | Unburned | Low-severity burned | High-severity burned |
| Production (lbs/ac) (SE in parentheses) | |||
| 1972 | no data | 7.0(1.7) | 2.2(1.6) |
| 1974 | 8.9(2.6) | 22.9(6.4) | 2.0(1.1) |
| 1980 | 29.8(23.1) | 3.3(1.2) | 1.2(0.6) |
| 2002 | trace | 0.4(0.4) | 0.1(0.1) |
| 2003 | 2.6(1.1) | 2.4(0.6) | 0.8(0.3) |
Variable responses to recovery following fire could be the result of pre- and post-fire weather, land use, or other conditions (Table 5). Effects of post-fire grazing were evaluated following a 1989 fall prescribed fire in a quaking aspen-conifer stand on the Manti-La Sal National Forest. Cover of American vetch was lowest on burned plots being grazed by cattle and big game and greatest on burned plots protected from both cattle and big game. Cover differences were greatest 15 years after the fire. This study evaluated primarily severely burned areas where all overstory trees were killed (Table 5; Walker et al. 2015).
Table 5. Cover of American vetch on plots in severely burned aspen-conifer stand on the Manti-La Sal National Forest that were protected from grazing, grazed by cattle, big game, or both. No species-level statistical analysis (Walker et al. 2015).
| Year | No grazing | Cattle | Big game | Both |
| Cover (%) of American vetch | ||||
| 1994 | 2 | 1 | 2 | 1 |
| 1999 | 5 | 4 | 4 | 5 |
| 2005 | 13 | 10 | 9 | 3 |
Wildlife And Livestock Use
Throughout its growing season American vetch is an important forage species for a variety of wildlife species from grizzly bears (Ursus arctos horribilis) to bees (Merrill et al. 1999). It is also highly palatable to cattle, sheep, and horses (Craighead et al. 1963).
Large mammals. Studies report that American vetch is an important food for grizzly bears (Merrill et al. 1999), bison (Bos bison) (Soper 1941), bighorn sheep (Ovis canadensis) (Courtemanch and Kauffman 2008), elk (Cervus canadensis), mule deer (Odocoileus hemionus), and white-tailed deer (O. virginianus) (Craighead et al. 1963; Thill et al. 1983). Black bear (U. americanus) food habits were evaluated from scat collected from the foothills of the Rocky Mountains in Alberta in 1984 and 1985. Frequency of American vetch in scat collected ranged from 63 to 89% in spring, 36 to 57% in early summer, and 9 to 21% in late summer (Holcroft and Herrero 1991). American vetch is considered a principal food of mule deer, white-tailed deer, and elk in Arizona’s mixed conifer forests (Thill et al. 1983). On the Uinta National Forest in Utah, American vetch made up just 1% of total vegetation but 4% of total utilization by mule deer (Smith 1949). In antelope bitterbrush (Purshia tridentata) rangelands in west-central Utah, American vetch comprised 24.4% of mule deer diets (by weight) in May and 10.9% in June. It was not eaten from July through September (Austin and Urness 1983). In feeding observations of tame mule deer and elk in aspen woodlands and clearcuts near Farmington, Utah (Collins and Urness 1983), significantly more American vetch was eaten by elk than deer in the woodlands but equally in the clearcut. Dry weight production of American vetch was about 13 lb/ac (15 kg/ha) greater in the clearcut than in the woodland. Forage consumption rates on American vetch averaged 0.69 g/min for elk and 0.24 g/min for deer (P < 0.05) in the woodland. Forage consumption rates were 0.14 g/min for both deer and elk in the clearcut (Collins and Urness 1983).
Small mammals. Many rodents feed on American vetch seeds and foliage (Hermann 1966). It makes up 0.5 to 2% of the diets of ground squirrels (Sciuridae) and moles (Talpidae) in Oregon and woodrats (Neotoma spp.) in California (Martin et al. 1951). American vetch was the most highly preferred plant of montane voles (Microtus montanus) when diets of four rodent species were compared in subalpine meadows in Grand County, Colorado. Based on stomach content analyses, American vetch made up 28% of July and 19% of August diets of montane voles. It was not highly preferred by the other rodents studied (Vaughan 1974). In a captive feeding trial, American vetch was considered one of the more edible meadow foods by both golden-mantled ground squirrels (Citellus lateralis lateralis) and least favored by chipmunks (Eutamias minimus consobrinus). Plants fed to the captive rodents were wild collected from their habitats in Gunnison County, Colorado (Carleton 1966). In other captive feeding trials to evaluate the selectivity of yellow-bellied marmots (Marmota flaviventris), American vetch leaves and stems were readily eaten. Marmots offered 5.5 oz (156 g) of American vetch ate 3.7 oz (105 g), when it was provided alongside dandelion (Taraxacum spp.) (Armitage 1979).
Birds. Both seeds and foliage of American vetch are eaten by birds (Hermann 1966). It is an important food plant for dusky grouse (Dendragapus obscurus) and grey partridge (Perdix perdix) (LeCount 1970; Weigand 1980). It makes up to 5% of dusky grouse and greater sage-grouse (Centrocercus urophasianus) diets and up to 2% of the diets of mourning doves (Zenaida macroura), ruffed grouse (Bonasa umbellus), sharp-tailed grouse (Tympanuchus phasianellus), ring-necked pheasants (Phasianus colchicus), greater prairie chickens (T. cupido), northern bobwhite (Colinus virginianus), scaled quail (Callipepla squamata), wild turkeys (Meleagris gallopavo), and song sparrows (Melospiza spp.) (Martin et al. 1951; Peterson 1969).
Frequency of American vetch was 11% in summer diets of grey partridge based on the analysis of 9 crops from birds in northeastern Teton County, Montana (Weigand 1980). American vetch and Nevada pea (Lathyrus lanszwertii var. leucanthus) were important foods for dusky grouse in the White Mountains of Arizona (LeCount 1970). Fall feeding habits were examined through the analysis of 99 crops. American vetch and Nevada pea, which were hard to distinguish and thus grouped, occurred in 71% of crops and made up 31% by volume. The plants made up 47% of adult male diets, and utilization was similar between adults and juveniles. Leaves, stems, flowers, and fruits were eaten, but leaves were preferred (LeCount 1970). In captive feeding trials, American vetch seed was eaten but not preferred by northern bobwhites. Among the 45 sand sagebrush (Artemisia filifolia)-mixed prairie plants presented to the birds, American vetch was one of the least preferred foods based on weight of seed consumed and percentage of seed in the diet (Thacker and Springer 2016).
Flowers and leaves are eaten by greater sage-grouse (Luna et al. 2018). American vetch was the fifth most preferred food of one- to two-week-old greater sage-grouse chicks based on crop and gizzard analyses of birds taken from big sagebrush-broom snakeweed (Gutierrezia sarothrae) habitats in central Montana in 1966 and 1968 (Peterson 1970). In big sagebrush habitat near Winnett, central Montana, American vetch made up a trace of juvenile greater sage-grouse crops. In the study area, American vetch made up a trace to 5% of the available vegetation (Peterson 1969).
Insects. American vetch is an important nectar and host plant for a variety of insects. Flowers and nectar production were studied in populations growing near Calgary, Alberta (Harder and Cruzan 1990). From the 43 inflorescences evaluated, nectar volume averaged 2.34 µL, nectar concentration was 36.9%, and sugar production averaged 1.01 mg. American vetch’s sugar production was among the highest of the Fabaceae and Ericacea species compared by Harder and Cruzan (1990). Floral nectar volume averaged 1.05 µL (n=503 flowers) and concentration averaged 24.8 mg/mL (n=285 flowers) for American vetch flowers evaluated in clearcut, 50% logged, and untreated boreal quaking aspen in northwestern Alberta (Pengelly and Cartar 2011). Floral nectar volume was second highest and floral nectar concentration was second lowest among the four forbs utilized by bumble bees that were evaluated. Nectar production rate for American vetch was significantly (P < 0.05) greater in 50% logged than unlogged sites (Pengelly and Cartar 2011).
In a study of the competition between bumblebees in mountain meadows of Gunnison Basin, Colorado, American vetch was visited mostly by white-shouldered bumble bees (50–75%) and yellow-fronted bumble bees (20–50%) based on observations made from late June to mid-August 1973 to 1975. Between 10 and 25% of visits were made by queens (Pleasants 1980). An oligolectic mason bee (Osmia iridis), forages almost exclusively on American vetch and Nevada pea (Keeler et al. 2021).
Various moths and butterflies use American vetch as a host and nectar plant. It is used as a larval host by western tailed-blues (Cupido amyntula). Eggs are laid on flowers and larvae emerge from fruits (James and Nunnallee 2011). In a breeding experiment, clouded sulphurs (Colias philodice eriphyle) were reared successfully on American vetch (Ellers and Boggs 2002). Mead’s sulphur (C. meadii) laid eggs on American vetch of the Rocky Mountain Biological Laboratory (RMBL) near Gunnison, Colorado (Ae 1958).
At the RMBL meadow, American vetch was used as a host plant by western tailed-blues, silvery blues (Glaucopsyche lygdamus), and Melissa blues (Plebejus melissa) based on observations made from June to August 1998 (Hughes 2000). At the same Colorado location, flower-feeding silvery blues commonly oviposited on American vetch. In laboratory rearings, American vetch successfully supported silvery blue larval growth (Breedlove and Ehrlich 1972). American vetch was used as a nectar plant by male and female Fender’s blue butterflies (P. icarioides fenderi) in Oregon’s Willamette Valley prairie remnants based on observations made from May 9 to June 15, 2020 (Thomas and Schultz 2016).
Several studies have focused on the importance of American vetch for sulfur butterflies (Colias spp.). Queen Alexandra’s sulphur (C. alexandra) works American vetch flowers “heavily” for nectar (Watt et al. 1974). In the East River Valley south of RMBL, American vetch averaged 19.3 µg sugar/flower when flowers were studied during Queen Alexandra’s sulphur feeding periods (Nielsen and Watt 1998). In studies in Gunnison County, Colorado, American vetch was the sixth most visited nectar plant by Queen Alexandra’s sulphur based on duration of seasonal use and number of observed visits (Watt et al. 1974).
Food plant selection by clouded sulphur, a legume feeder, was studied in a dry fescue-sagebrush community about 9 mi (14 km) from the RMBL (Stanton 1982). At this study site, legume populations were diverse and included clovers (Trifolium spp.), lupine (Lupinus spp.), American vetch, Nevada pea, and prostrate milkvetch (Astragalus miser var. decumbens). The probability that clouded sulphur oviposited after landing on American vetch was 0.424. Using data from all broods, American vetch was oviposited on twice as often and landed on 1.75 times as often than would be predicted from its abundance relative to the other legumes. In drought years, American vetch was landed on only four times and not oviposited on at all (Stanton 1982).
Livestock. Native vetches are highly palatable to domestic livestock, especially sheep, throughout the growing season (USDA FS 1937). It is considered excellent forage and used by all classes of livestock, including cattle and horses. It is known for its fattening qualities for sheep (Hermann 1966).
Cattle fed on American vetch to varying degrees in the Northern Plains. In shortgrass prairie winter range northwest of Great Falls, Montana, density of American vetch was high in cattle feces even though its canopy cover was low in the study area. This suggested that cattle selectively fed on American vetch (Kasworm et al. 1984). In Montana’s Missouri River Breaks, American vetch made up to 1% of total instances of annual cattle use. In habitat types evaluated for cattle use, American vetch was rare making up just a trace of cover (Mackie 1970).
American vetch is preferred by sheep and utilization can be heavy. On high-elevation summer range in southwestern Utah, American vetch did not occur in plots subjected to prolonged sheep grazing but reached production levels of 34 lbs/ac (38 kg/ha) in plots grazed lightly by horses and cattle (Bowns and Bagley 1986).
A study by McInnis et al. (1983) found that use rates and diet percentages can vary by the method selected to determine usage. In a dry meadow at Hall Ranch, eastern Oregon Agricultural Research Center, dry weight composition of American vetch in sheep diets was 6.5% by utilization, 0.2% by esophageal analysis, a trace by rumen analysis, and 0% by fecal analysis. Differences between the various methods were not significant (McInnis et al. 1983).
In studies where both cattle and sheep grazed or effects of cattle use and sheep use were compared, American vetch was lower on grazed than ungrazed plots. In subalpine range on Utah’s Wasatch Plateau, heavy cattle and sheep grazing were monitored and compared to ungrazed range for 32 to 35 years. Density of American vetch stems was 4 to 10 times greater on ungrazed plots than on plots heavily grazed by cattle. Stem density was 11 to 25 times greater on ungrazed plots than on plots heavily grazed by sheep (Ellison 1954). Utilization of American vetch was 93 to 99% during a study to compare the effects of grazing by sheep, cattle, or both on high-elevation summer range in Gambel oak and quaking aspen stands in southwestern Utah. There was no significant difference in utilization by year or grazing animal (Ruyle and Bowns 1985). In Douglas-fir-ponderosa pine forests at the University of Idaho’s Experimental Forest in Latah, County, American vetch was absent from grazed plots, but production was 1.2 lbs/ac (1.3 kg/ha) on ungrazed plots. Grazed plots were used heavily by sheep and cattle from 1945 to 1967 and by cattle in spring, summer, and fall from 1969 to 1978. The exclosure protecting vegetation from livestock but not from big game was constructed in the 1940s (Zimmerman and Neuenschwander 1984).
Nutritional Value
The nutrient content of American vetch was reported from several locations. In western rangelands, phosphorus content decreases over the growing season, averaging 0.4% in spring and 0.2% in summer (Welch 2004). American vetch plants collected from rough fescue grasslands in southwestern Alberta were among the species with the highest digestible protein. In vitro digestibility of American vetch was averaged for plants at leaf stage, heading, and ripe seed stage of growth (Table 6; Bezeau and Johnston 1962).
Table 6. Nutritive value of American vetch averaged from leaf stage to mature seed stage in grasslands in southwestern Alberta (Bezeau and Johnston 1962).
| Life stage | Cellulose (%) | Digestible coefficient of cellulose (%) | Digestible protein (%) | Nutritive value index |
| Leaf stage | 28.4 | 54.1 | 13.0 | 63.3 |
| Heading | 26.9 | 40.9 | 7.2 | 45.9 |
| Ripe seed | 28.9 | 40.5 | 5.4 | 45.4 |
Protein levels were high for American vetch plants collected in summer 1977 from sagebrush communities in central Washington. In this study area where the distribution of American vetch was limited, elk utilization was 0.29% in June and July and 0.08% in August based on fecal analysis (Table 7; Boltz 1979).
Table 7. Nutritional value of American vetch plants collected in early summer in central Washington (Boltz 1979).
| Timing | Gross moisture | Oven-dry moisture | Crude protein | Total ash | Cell solubles | Hemicellulose | Cellulose | Lignin-cutin |
| ————-%————– | ||||||||
| June 22 | 70.6 | 7.3 | 29.4 | 8.6 | 66.4 | 6.1 | 21.8 | 5.3 |
| July 11 | 70.0 | 6.0 | 22.5 | 8.6 | 61.9 | 7.3 | 23.4 | 6.2 |
Chemical composition was similar for American vetch plants collected from Cascade golden-mantled ground squirrel (Spermophilus saturatus) habitats near Fish Lake in Chelan County, Washington (Table 8; Cork and Kenagy 1989).
Table 8. Chemical composition of American vetch plants collected near Fish Lake in Chelan County, Washington (Cork and Kenagy 1989).
| Total nitrogen | Ash | Acid-detergent fiber | Cellulose | Lignin |
| ———-% total dry matter———- | ||||
| 2.55 | 7.8 | 28.4 | 19.5 | 8.9 |
Ethnobotany
Indigenous peoples use American vetch for food, as medicine, and in ceremonies. Young shoots and tender pods and seeds are eaten (Craighead et al. 1963; Andersen and Holmgren 1996; Kirk and Belt 2010). Poultices of leaves are used to treat spider bites, and an infusion of leaves is used as an eyewash and a wash in sweathouses. It is considered a panacea, aphrodisiac, and good luck charm. Some tribes delivered American vetch smoke to horses to increase endurance (Moerman 2003; Kirk and Belt 2010).
The Ramah refer to American vetch as a life medicine and use an infusion of the plant as an eyewash (Vestal 1952). Iroquois women use a root infusion as a love medicine. The Squaxin use an infusion of crushed leaves in a bath to relieve soreness (Moerman 2003).
A plant use evaluation by Chesnut (1902) reported that the Yuki, Pomo, and Yokai tribes as well as other tribes living in or visiting Mendocino County, California, used American vetch as fodder for livestock. They ate young stems raw and more mature stems and leaves cooked. Stout American vetch roots were used as fastening cords, and a small bunch of roots were kept in the pocket for good luck when gambling (Chesnut 1902).
Horticulture
American vetch has potential for use in low-maintenance landscapes like roadways, rest areas, campgrounds, or other low maintenance landscapes. It provides pollinator food and habitat and forage for other wildlife. The bright purple to pink flowers are attractive, but the gangly growth form may not be a good fit for heavily manicured landscapes. However, its tolerance of sun and shade conditions make it a versatile addition to more wild-kept yards. The spreading growth habit and extensive root system make it useful for stabilizing slopes. American vetch is available as seed from native nurseries or online shops.
Revegetation Use
American vetch has many characteristics that make it useful for revegetation. It is drought tolerant and grows in medium- to coarse-textured soils at sites receiving at least 10 in (250 mm) of annual precipitation (Kirk and Belt 2010; Allen and Tilley 2014; Ogle et al. 2014). It grows in sun and partially shaded habitats in nearly all western Major Land Resource Areas (Kirk and Belt 2010; Taliga 2011; Eldredge et al. 2013). Once established it grows rapidly, and its spreading growth habit and well-developed root system makes it good for soil stabilization (Ogle et al. 2017). As a nitogen-fixing species, it can be used to remediate disturbed rangelands by increasing available nitrogen (Allen and Tilley 2014). It provides excellent wildlife and livestock forage and is important to many pollinators (Ogle et al. 2017).
American vetch tolerates ruderal conditions and often colonizes degraded sites when a nearby seed source is available (Fig. 8). In subalpine habitats in west-central Alberta, it grew on coal mine spoil heaps visited about 25 years after abandonment. The spoils were primarily coal, siltstone, and shale, neutral to slightly acidic with a loamy sand to sandy loam texture (Russell 1980). American vetch established as a volunteer on reclaimed bentonite mine spoils in Carter County, Montana. By 5 to 12 years after mine spoils were contoured, spread with topsoil, and seeded with wheatgrasses (Agropyron spp.) and sweetclover (Melilotus officinalis), American vetch cover was the same (0.2%) in undisturbed big sagebrush-grassland and reclaimed mine spoils (Sieg et al. 1983). American vetch volunteered on a coal mine site in northeastern Wyoming, where it was a dominant forb in neighboring grasslands. Reclamation of the spoils included slope contouring, backfill ripping, topsoil replacement, fertilizing, and seeding with non-native and native species but no American vetch. Vegetation on the reclaimed spoils was evaluated 2 to 4 years following seeding (Schladweiler et al. 2005).

Figure 8. American vetch growing along a mowed roadside. Photo: M. Lavin, MSU.
Developing A Seed Supply
For restoration to be successful, the right seed needs to be planted in the right place at the right time. Coordinated planning and cooperation is required among partners to first select appropriate species and seed sources and then properly collect, grow, certify, clean, store, and distribute seed for restoration (PCA 2015).
Developing a seed supply begins with seed collection from native stands. Collection sites are determined by current or projected revegetation requirements and goals. Production of nursery stock requires less seed than large-scale seeding operations, which may require establishment of agricultural seed production fields. Regardless of the size and complexity of any revegetation effort, seed certification is essential for tracking seed origin from collection through use (UCIA 2015).
Seed Sourcing
Because empirical seed zones are not currently available for American vetch, generalized provisional seed zones developed by Bower et al. (2014) may be used to select and deploy seed sources. These provisional seed zones identify areas of climatic similarity with comparable winter minimum temperature and aridity (annual heat to moisture index). In Figure 9, Omernik Level III Ecoregions (Omernik 1987) overlay the provisional seeds zones to identify climatically similar but ecologically different areas. For site-specific disturbance regimes and restoration objectives, seed collection locations within a seed zone and ecoregion may be further limited by elevation, soil type, or other factors.
The Western Wildland Environmental Threat Assessment Center’s (USFS WWETAC 2017) Threat and Resource Mapping (TRM) Seed Zone application provides links to interactive mapping features useful for seed collection and deployment planning. The Climate Smart Restoration Tool (Richardson et al. 2020) can also guide revegetation planning, seed collection, and seed deployment, particularly when addressing climate change considerations.
Occurrence Map
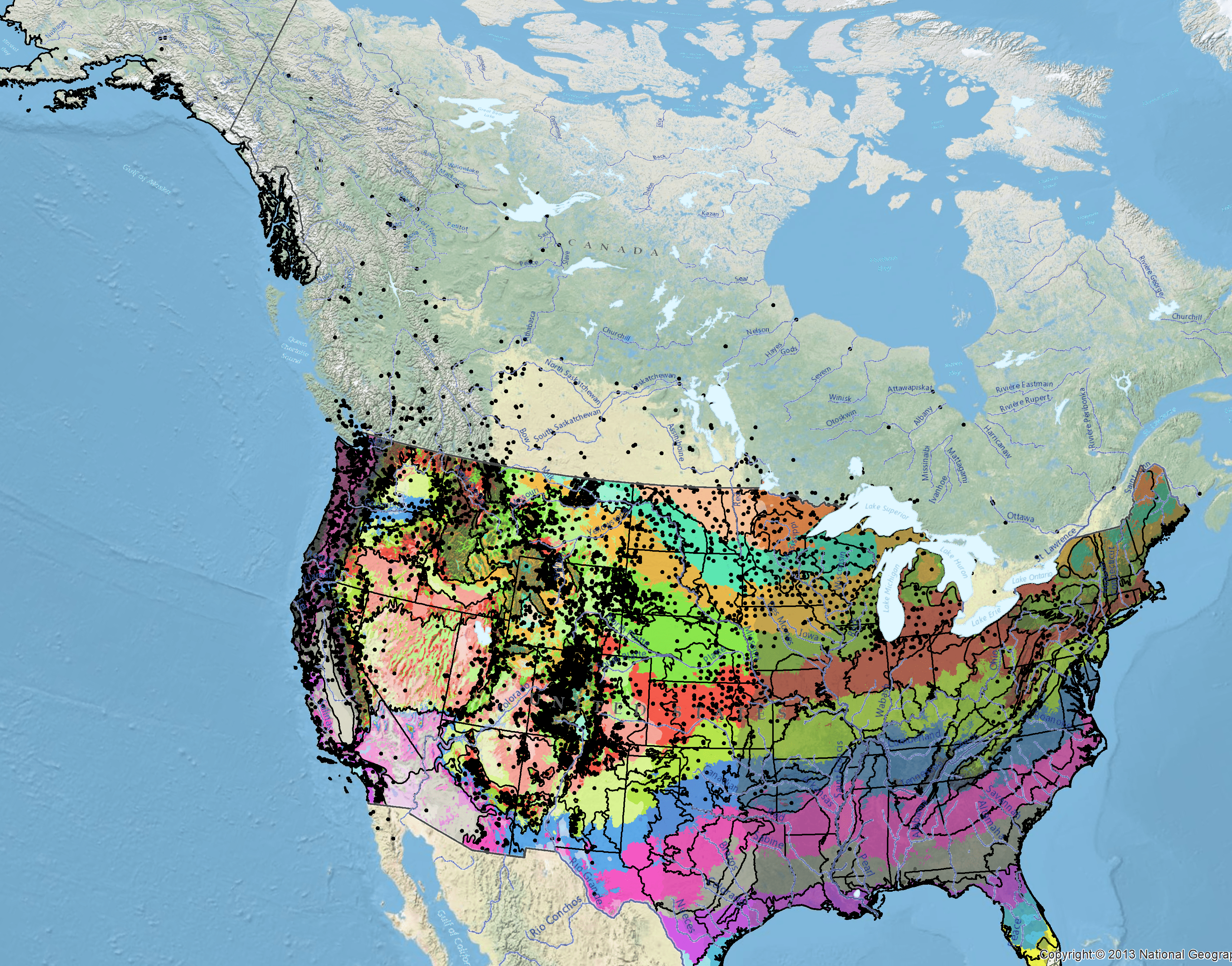
Figure 9. Distribution of American vetch (black circles) based on geo–referenced herbarium specimens and observational data from 1881–2016 (CPNWH 2020; SEINet 2020; USDI USGS 2020). Generalized provisional seed zones (colored regions) (Bower et al. 2014) are overlain by Omernik Level III Ecoregions (black outlines) (Omernik 1987; USDI EPA 2018). Interactive maps, legends, and a mobile app are available (USFS WWETAC 2017; www.fs.fed.us/wwetac/threat–map/TRMSeedZoneMapper2.php?). Map prepared by M. Fisk, USDI USGS.
Releases
As of 2022, there were no American vetch germplasm releases. Seed source selection should use information about local climate, local pests, and intended use (Allen and Tilley 2014).
Wildland Seed Collection
Wild American vetch seed is collected by hand typically in July in the West, but seeds can be found as late as September. Harvesting can be labor intensive given the often twining and sprawling growth habit (Fig. 10) (Gould et al. 2013; BLM SOS 2020).
Wildland Seed Certification
Verification of species and tracking of geographic source is necessary whether wildland seed is collected for immediate project use or as stock seed for cultivated increase. This official Source Identification process can be accomplished by following procedures established by the Association of Official Seed Certifying Agencies (AOSCA) Pre-Variety Germplasm Program (Young et al. 2020; UCIA 2015). Wildland seed collectors should become acquainted with state certification agency procedures, regulations, and deadlines in the states where they collect.
If wildland-collected seed is to be sold for direct use in ecological restoration projects, collectors must apply for Source-Identified certification prior to making collections. Pre-collection applications, site inspections, and species and seed amount verification are handled by the AOSCA member state agency where seed collections will be made (see listings at AOSCA.org).
If wildland seed collected by a grower or private collector is to be used as stock seed for planting cultivated seed fields or for nursery propagation (See Agricultural Seed Field Certification section), detailed information regarding collection site and collecting procedures must be provided when applying for certification. Photos and herbarium specimens may be required. Germplasm accessions acquired within established protocols of recognized public agencies, however, are normally eligible to enter the certification process as stock seed without routine certification agency site inspections. For contract grow-outs, however, this collection site information must be provided to the grower to enable certification.
Collection Timing
Mature seed production is influenced by location, elevation, and local weather (Munz and Keck 1973; Barneby 1989; Ogle et al. 2017). While seed is commonly ready for harvest in July in the West, it can be found as late as September (Gould et al. 2013; BLM SOS 2020). The Bureau of Land Management’s Seeds of Success crews reported just four American vetch harvests. Collections were made in California, Utah, and Colorado in 4 separate years. The earliest harvest was made on July 1, 2004, in Salt Lake County, Utah, at 5,949 ft (1,813 m) elevation. The latest harvest was made on July 21, 2011, at a 6,185-ft (1,885 m) elevation site in El Dorado County, California (BLM SOS 2020).
Collection Methods
Crews collected American vetch by clipping or stripping inflorescences into paper bags (Gould et al. 2013).

Figure 10. American vetch dry seed pods, ready for harvest. Photo: BLM NV030, SOS.
Several collection guidelines and methods should be followed to maximize the genetic diversity of wildland collections: 1) collect seed from a minimum of 50 randomly selected plants; 2) collect from widely separated individuals throughout a population without favoring the most robust or avoiding small stature plants; and 3) collect from all microsites including habitat edges (Basey et al. 2015). General collecting recommendations and guidelines are provided in online manuals (e.g., ENSCONET 2009; USDI BLM SOS 2021). It is critical that wildland seed collection does not impact the sustainability of native plant populations. Collectors should take no more than 20% of the viable seed available at the time of harvest (USDI BLM SOS 2021). Additionally, care must be taken to avoid the inadvertent collection of weedy species, particularly those that produce seeds similar in shape and size to those of American vetch.
Post-Collection Management
Seed collections should be stored in breathable containers and protected from rodents until they can be thoroughly dried and cleaned. Insects present in seed harvests can be controlled by freezing or adding mothballs to the collection.

Figure 11. Single, dry American vetch seed pod and a single mature seed. Photo: Chicago Botanical Garden-4188.
Seed Cleaning
Seed can be cleaned by first crushing and screening the pods. Winnowing removes the resulting chaff produced. Seed is then screened using an 8.5/64-in, round top screen and 1/15-in, round bottom screen (Gould et al. 2013).
Seed Storage
American vetch seed is orthodox. Seed retained 70% viability after dry seed was stored for 4 months at 68 °F (-20 °C) (SER, INSR, RBGK, SID 2023). Long-term drying does not harm viability or germination potential (Griswold 1936).
Seed Testing
The Association of Official Seed Analysts (AOSA) provide a tetrazolium chloride (TZ) viability test for vetch species (AOSA/SCST 2010). Seed is pre-conditioned by imbibing it at 77 °F (20–25 °C) on moist towels for two hours then soaking it in water for another two hours or leaving it on moist towels overnight. Record the percentage of hard seed present after imbibing. Seeds are then cut longitudinally to bisect the embryo axis and exposed to 0.1 to 1% TZ for one hour at 86 to 95 °F (30–35 °C). Seeds with the entire embryo evenly stained or with minimal unstained or dark red stained portions are viable (AOSA/SCST 2010). There is no AOSA rule for testing germination.
Germination Biology
American vetch seed has a hard seed coat, and germination requires scarification. Germination percentages and rates can be improved with stratification, especially using alternating warm-cold or moist-dry cycles, but stratification is not as effective as scarification (as reported above) (Griswold 1936; Love and Akins 2020; SER, INSR, RBGK, SID 2023).
Germination of 65 to 100% was reported for seed scarified by partial seed coat removal with scalpel, by puncture, or mechanical scarification. Germination trials conducted by Royal Botanical Gardens (SER, INSR, RBGK, SID 2023) reported the highest germination (70%) after 35 days when scarified seed was incubated on a 1% agar medium at 68 °F (20 °C) and exposed to an 8/16 hr light/dark cycle. Germination percentages were lower (65%) for scarified seed incubated at 59 °F (15 °C) and even lower for unscarified seed. Scarification was accomplished by partial seed coat removal with a scalpel (SER, INSR, RBGK, SID 2023). Germination was 100% and occurred within 2 to 6 days for seeds with holes ground through the seed coat (Love and Akins 2020). A review by Gould et al. (2013) reported 78% within 3 to 7 days for mechanically scarified seed.
Germination percentages were often 40% or less for unscarified seed (Griswold 1936; Ahlgren 1979; Bjugstad and Whitman 1989). Alternating cold-warm temperatures or dry-moist conditions improved germination somewhat (up to 75%) (Griswold 1936; Love and Akins 2020) but failed to produce the germination rates or percentages obtained through scarification.
There are likely ecotypic differences in germination requirements. Mackenzie and Naeth (2017) reported that most of the seed collected in northeastern Alberta germinated before the end of a six-week stratification period at 36 to 39 °F (2–4 °C). The wild-collected seed was 90% viable and stored in the dark at room temperature until testing. For non-stratified seed, treatments of smoke water, potassium nitrate, and smoke water + potassium nitrate did not improve germination, which was close to 20% for all treatments (Mackenzie and Naeth 2017).
Wildland Seed Yield And Quality
Post-cleaning seed yield and quality of two seed lots collected in the Intermountain Region are provided in Table 9 (USFS BSE 2017). The results indicate that American vetch seed can generally be cleaned to high levels of purity and seed fill and that viability of fresh seed was high.
Numbers of seeds/lb reported elsewhere (24,500–46,409 PLS/lb [54,012–102,313 PLS/kg]) were often much greater than those in Table 9 (Stevens 1957; Wasser and Shoemaker 1982; Hassell et al. 1998; Winslow et al. 2009; Gould et al. 2013; USFS GBNPP 2014; SER, INSR, RBGK, SID 2023) In a review, Gould et al. (2013) reported 27,216 PLS/lb (60,000 PLS/kg) (Gould et al. 2013). While bulk or clean seed weights may be low based on the two seed lots cleaned by the USDA Forest Service, Bend Seed Extractory, the purity was very near that reported for a seed lot used in restoration of a site near Pinedale, Wyoming (96% purity) (Winslow et al. 2009).
Table 9. Seed yield and quality of American vetch seed lots collected in the Intermountain region, cleaned by the Bend Seed Extractory, and tested by the Oregon State Seed Laboratory or the USDA Forest Service National Seed Laboratory (USFS BSE 2017).
| Seed lot characteristic | Mean | Range | Samples (no.) |
| Bulk weight (lbs) | 0.49 | 0.26–0.73 | 2 |
| Clean weight (lbs) | 0.13 | 0.11–0.15 | 2 |
| Clean–out ratio | 0.3 | 0.21–0.42 | 2 |
| Purity (%) | 99 | 99–99 | 2 |
| Fill (%)¹ | 91 | 82–99 | 2 |
| Viability (%)² | 96 | 96 | 1 |
| Seeds/lb | 14,925 | 14,000–15,850 | 2 |
| Pure live seeds/lb | 13,306 | 13,306 | 1 |
¹100 seed X–ray test
²Tetrazolium chloride test
Marketing Standards
Wasser and Shoemaker (1982) indicates that American vetch seed purity should be 95% or better, germination should reach at least 60%, and PLS should be 57% or better (Wasser and Shoemaker 1982). Acceptable seed purity, viability, and germination specifications vary with revegetation plans. Purity needs are highest for precision seeding equipment used in nurseries, while some rangeland seeding equipment handles less clean seed quite well.
Agricultural Seed Production
If American vetch has been grown for seed production in the US, reports were lacking in the literature. Burton and Burton (2002) indicate that seed production fields were grown in northwestern British Columbia, but we found no additional information on production there.
Agricultural Seed Certification
In order to minimize genetic changes in specific accessions of native species when increased in cultivated fields, it is essential to track the geographic source and prevent inadvertent hybridization or selection pressure. This is accomplished by following third party seed certification protocols for Pre-Variety Germplasm (PVG) as established by the Association of Official Seed Certification Agencies (AOSCA). AOSCA members in the U.S., Canada, and other countries administer PVG requirements and standards that track the source and generation of planting stock. Field and cleaning facility inspections then monitor stand establishment, proper isolation distances, control of prohibited weeds, seed harvesting, cleaning, sampling, testing, and labeling for commercial sales (Young et al. 2020; UCIA 2015).
Seed growers apply for certification of their production fields prior to planting and plant only certified stock seed of an allowed generation (usually less than four). The systematic and sequential tracking through the certification process requires preplanning, knowing state regulations and deadlines, and is most smoothly navigated by working closely with state certification agency personnel. See the Wildland Seed Certification section for more information on stock seed sourcing.
Seeding
In a review, Allen and Tilley (2014) report a full-stand seeding rate of approximately 25 seeds/ft² (269/m²) or 33 PLS lbs/ac (36 PLS kg/ha). Seeding in the fall at a depth of 1 to 2 in (2.5–5 cm) is recommended. Unscarified seed germinates in about 2 weeks. Scarified seed will germinate in 3 to 7 days. Scarification was said to speed up germination but not affect germination percentage of fall seedings (Allen and Tilley 2014).
American vetch can be propagated vegetatively from rhizome cuttings (Currah et al. 1983).
Establishment And Growth
In controlled studies, American vetch seed germinated in sterilized soils, but germination in soils lacking rhizobia was much later than that in soils with rhizobia. This suggests that establishment is slower if rhizobia are dormant at the time of germination and early seedling growth (Keeler and Rafferty 2018). Rhizobium leguminosarum is considered a possible microsymbiont in restoration seedings (Graham 2005).
Pest Management
Wasser and Shoemaker (1982) note that American vetch stands (foliage and seeds) are susceptible to insect and rodent damage. Leaf-tier (Cnephasia longana), an insect pest on cereal crops, feeds on American vetch in its larval stage (Gilligan 2019). Many (>20) fungal species use American vetch as a host in wildlands (Farr and Rossman 2017). Botrytis fungal species are blamed for poor natural reseeding of vetch species in pastures (Kirk and Belt 2010).
Seed Harvesting
Because of its low, sprawling, and twining growth habit, vacuum harvesting is not practical (Allen and Tilley 2014). The twining growth habit suggests that American vetch could be grown with another structurally supportive plant to elevate the seed pods and improve mechanical harvesting. Any species seeded with American vetch for support should have significantly dissimilar seed size and weight for easy separation (Allen and Tilley 2014).
Nursery Practice
American vetch was grown successfully in a greenhouse study evaluating the growth of species being considered for use in oil sands mine reclamation in northern Alberta (Buss et al. 2018). The study tested performance of American vetch, northern bedstraw, and scentless false mayweed (Tripleurospermum perforatum) (nonnative) in three soil types and with and without fertilization. The species were seeded in small plugs (0.8 × 0.8 in [2 × 2 cm]) filled with a peat potting mix. Three weeks after seeding, American vetch seedlings were transplanted into experimental pots filled with a forest floor mineral mix (FFMM), peat-mineral mix (PMM), or a layered mix (1/3 FFMM on top of 2/3 PMM). At the time of transplanting, American vetch plug seedlings averaged 2.5 in (6.4 cm) tall. Pots that were fertilized received liquid NPK 30-10-10 once each week for 4 weeks for a total amount of 89 lbs/ac (100 kg/ha). Pots were kept in a greenhouse operating on a 18 to 6 hour light-dark cycle and a 75:48 °F (24:9 °C) temperature cycle. Aboveground biomass of American vetch was not different between soil or fertilization treatments, but belowground biomass was significantly greater in layered than PMM soils (P < 0.05) and fertilization significantly reduced belowground growth (P = 0.020). No American vetch plants died in the 13-week experiment, and it had the highest per gram water usage of all species evaluated. Researchers concluded that American vetch was a good candidate for establishing and might outcompete non-native species on low resource soils like PMM (Buss et al. 2018).
Wildland Seeding And Planting
American vetch is a good choice for revegetation of wildlife and pollinator habitat (Ogle et al. 2017). Its spreading growth above and below ground makes it useful for soil stabilization. It is drought tolerant, adds available nitrogen to the soil, and tolerates ruderal conditions making it useful on dry degraded sites (Eldredge et al. 2013; Ogle et al. 2014).
Ecotypes are adapted to medium- to coarse-textured neutral to somewhat acidic soils at sites receiving 9 to 50 in (230–1,270 mm) of annual precipitation. The recommended full seeding rate is 33 PLS lbs/ac (36 PLS kg/ha), although it would typically make up less than 10% of a seeding mix. Recommended seeding depth ranges from 0.25 to 2 in (0.6–5 cm) (Eldredge et al. 2013; Ogle et al. 2014, 2017). Shallower seeding (0.5 in [1.2 cm]) is recommended for moist, course-textured soils and deeper seeding (1.5 in [3.8 cm]) is recommended for dry, fine-textured soils (Wasser and Shoemaker 1982). Grazing should be deferred for at least two growing seasons to allow for full establishment (Allen and Tilley 2014). Based on revegtation efforts in Alberta, Gould et al. (2013) recommends a seeding rate of 330–490 seeds/ft (100–150/m) and a seeding depth of 0.4 in (1 cm).
For revegetation of disturbed sites, Wasser and Shoemaker (1982) suggest drill seeding American vetch 0.5 in (1.2 cm) deep in moist or course-textured soils and up to 1.5 in (3.8 cm) in dry or fine-textured soils at a rate of 3 to 8 lbs/ac (3.4–11 kg/ha). Higher seeding rates are needed for harsh (eroded, saline) sites. Scarified seed can be planted early in the growing season and can result in quick germination and seedling establishment. Unscarified seed should be fall planted to break seed dormancy. As a large-seeded species, American vetch is a good choice for aerial seeding on rough mountainous terrain (Wasser and Shoemaker 1982).
American vetch has been established deliberately and voluntarily in various revegetation projects. Emergence of American vetch was compared on three sites within reclaimed oil sand mines using fall and spring broadcast seeding in northeastern Alberta (Smreciu and Gould 2015). Seedlings emerged from all three sites, but emergence was significantly (P < 0.05) greater on the Mildred Lake site (ML; 4%) than the Aurora (A; 0.57%) or Athabasca River sites (AR; 0.11%). Emergence was not significantly different between the timing of seeding (fall vs. spring). The ML site was a north-facing slight slope, had high levels of weed competition, clay to clay loam soils with a pH of 7.2 to 7.8 and average soil temperature of 56.3 °F (13.5 °C). The A site was a steep south-facing slope with sparse cover on loamy sand to sandy loam soils with a pH of 7.4 to 7.6 and average temperature of 61.3°F (16.3 °C). The AR site was nearly flat, had sandy loam to sandy clay loam soils with average pH of 7.29 and soil temperature of 59.4 °F (15.2 °C). AR was the only site that was plowed prior to sowing (Smreciu and Gould 2015).
A study at Grasslands National Park in southern Saskatchewan suggests American vetch may be useful in diversifying nonnative crested wheatgrass (Agropyron cristatum) stands. Heidinga and Wilson (2002) evaluated the cover of native plants along a transect from an old field planted to crested wheatgrass in the 1940s or 50s to a native prairie dominated by needle and thread (Hesperostipa comata) and blue grama (Bouteloua gracilis) where crested wheatgrass was rare. American vetch cover decreased with increasing cover of crested wheatgrass but not significantly (P > 0.05, n=118). Seven of the nine native species evaluated were significantly reduced by increasing abundance of crested wheatgrass (Heidinga and Wilson 2002).
In Fargo, North Dakota attempts to encouraging native forb growth by reducing competition failed to increase abundance of American vetch. Effects of a broadleaf herbicide aimed at reducing abundance or vigor of leafy spurge (Euphorbia esula) and Canada thistle (Cirsium arvense) were evaluated following a July broadcast treatment of aminocyclopyrachlor (140 g/ha). Cover of American vetch was significantly lower on treated (<0.1%) than untreated plots (0.7%) evaluated 10 months and 14 months after treatment (P < 0.05) (Thilmony and Rodney 2017).
In revegetation trials conducted on well-pads about 30 mi (48 km) south of Pinedale, Wyoming, American vetch establishment and vigor was poor (Winslow et al. 2009). American vetch was part of a drill seed mix seeded at a rate of 30 PLS/ft² (320/m²) on October 2005. Soil was ripped, firmed, and smoothed before seeding 24 forbs in test plots. In 2007, American vetch density was 0.04 plants/ft² (0.4/m²) and average height was 0.25 in (0.6 cm). Researchers rated it lowest for vigor and percent stand establishment among all forbs tested. The American vetch seed lot used had 96% purity and 87% germination (Winslow et al. 2009; Winslow et al. n.d.).
Many other studies monitoring the revegetation of mine sites found American vetch establishing voluntarily on spoils. (See also the Revegetation Use section).
Acknowledgements
Funding for Western Forbs: Biology, Ecology, and Use in Restoration was provided by the USDI BLM Great Basin Native Plant Materials Ecoregional Program through the Great Basin Fire Science Exchange. Great thanks to the chapter reviewers: Laura Fischer Walter, Tallgrass Prairie Center and Stanley Kitchen, USDA Forest Service.
This research was supported in part by the USDA Forest Service, Rocky Mountain Research Station. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA or U.S. Government determination or policy.
Literature Cited
Abella, S.R.; Covington, W.W. 2006. Vegetation-environment relationships and ecological species groups of an Arizona Pinus ponderosa landscape, USA. Plant Ecology. 185(2): 255-268.
Ae, S.A. 1958. Comparative studies of developmental rates, hibernation, and food plants in North American Colias (Lepidoptera, Pieridae). The American Midland Naturalist 60(1): 84-96.
Ahlgren, C.E. 1979. Buried seed in the forest floor of the Boundary Waters Canoe Area. Minnesota Forestry Research Notes No. 271. St. Paul, MN: University of Minnesota, College of Forestry. 4 p.
Albertson, F.W.; Weaver, J.E. 1944. Nature and degree of recovery of grassland from the great drought of 1933 to 1940. Ecological Monographs. 14(4): 394-479.
Alexander, B.G., Jr.; Fitzhugh, E.L.; Ronco, F., Jr.; Ludwig, J.A. 1987. A classification of forest habitat types of the northern portion of the Cibola National Forest, New Mexico. Gen. Tech. Rep. RM-143. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 35 p.
Allen, J.; Tilley, D. 2014. Plant guide for American vetch (Vicia americana). Aberdeen, ID: U.S. Department of Agriculture, Natural Resources Conservation Service, Plant Materials Center. 3 p.
Anderson, B.A.; Holmgren, A.H. 1976. Mountain plants of northeastern Utah. Logan, UT: Utah State University, Extension Services. 148 p.
Anderson, H.G.; Bailey, A.W. 1980. Effects of annual burning on grassland in the aspen parkland of east-central Alberta. Canadian Journal of Botany. 58: 985-996.
Anderson, M.L.; Bailey, A.W. 1979. Effect of fire on a Symphoricarpos occidentalis shrub community in central Alberta. Canadian Journal of Botany. 57: 2820-2823.
Archibold, O.W.; Ripley, E.A.; Delanoy, L. 2003. Effects of season of burning on the microenvironment of fescue prairie in central Saskatchewan. The Canadian Field-Naturalist. 117(2): 257-266.
Armitage, K.B. 1979. Food selectivity by yellow-bellied marmots. Journal of Mammalogy. 60(3): 628-629.
Armour, C.D.; Bunting, S.C.; Neuenschwander, L.F. 1984. Fire intensity effects on the understory in ponderosa pine forests. Journal of Range Management. 37(1): 44-49.
Association of Official Seed Analysts [AOSA]. 2010. AOSA/SCST Tetrazolium testing handbook. Contribution No. 29. Lincoln, NE: Association of Official Seed Analysts.
Austin, D.D.; Urness, P.J. 1983. Summer use of bitterbrush rangelands by mule deer. In: Tiedemann, A.R.; Johnson, K.L., comps. In: Proceedings- Research and management of bitterbrush and cliffrose in western North America; 1982 April 13-15; Salt Lake City, UT. Gen. Tech. Rep. INT-152. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: 203-212.
Barneby, R.C. 1989. Intermountain Flora Volume 3, Part B: Fabales. In: Cronquist, A.; Holmgren, A.H.; Holmgren, N.H.; Reveal, J.L.; Holmgren, P.K., eds. Intermountain flora: Vascular plants of the Intermountain West, U.S.A. Bronx, NY: The New York Botanical Garden. 279 p.
Basey, A.C.; Fant, J.B.; Kramer, A.T. 2015. Producing native plant materials for restoration: 10 rules to collect and maintain genetic diversity. Native Plants Journal. 16(1): 37-53.
Bataineh, A.L.; Oswald, B.P.; Bataineh, M.M.; Williams, H.M.; Coble, D.W. 2006. Changes in understory vegetation of a ponderosa pine forest in northern Arizona 30 years after a wildfire. Forest Ecology and Management. 235(1): 283-294.
Bezeau, L.M.; Johnston, A. 1962. In vitro digestibility of range forage plants of the Festuca scabrella association. Canadian Journal of Plant Science. 42(4): 692-697.
Bingham, M.A.; Biondini, M. 2009. Mycorrhizal hyphal length as a function of plant community richness and composition in restored northern tallgrass prairies (USA). Rangeland Ecology and Management. 62(1): 60-67.
Biondini, M.E., Patton, B.D., and Nyren, P.E. 1998. Grazing intensity and ecosystem processes in a northern mixed-grass prairie, USA. Ecological Applications. 8(2): 469-479.
Bjugstad, A.J.; Whitman, W.C. 1989. Promising native forbs for seeding on mine spoils. In: Walker, D.G.; Powter, C.B.; Pole, M.W., comps. Proceedings of the conference: Reclamation, a global perspective; 1989 August 27-31; Calgary, AB. Edmonton, AB: Alberta Land Conservation and Reclamation Council: 255-262.
Blackwell, L.R. 2006. Great Basin wildflowers. Falcon Guides. Guilford, CT: Globe Pequot Press. 281 p.
Boltz, M.J. 1979. Impacts of prescribed burns and clearcuts upon summer elk food habitats, diet quality, and distribution in central Washington. Pullman, WA: Washington State University. Thesis. 129 p.
Bork, E.W.; Adams, B.W.; Willms, W.D. 2002. Resilience of foothills rough fescue, Festuca campestris, rangeland to wildfire. The Canadian Field Naturalist. 116(1): 51-59.
Bower, A.D.; St. Clair, J.B.; Erickson, V. 2014. Generalized provisional seed zones for native plants. Ecological Applications. 24(5): 913-919.
Bowns, J.E.; Bagley, C.F. 1986. Vegetation responses to long-term sheep grazing on mountain ranges. Journal of Range Management. 39(5): 431-434.
Breedlove, D.E.; Ehrlich, P.R. 1972. Coevolution: Patterns of legume predation by a Lycaenid butterfly. Oecologia. 10(2): 99-104.
Burkle, L.; Irwin, R. 2009. The importance of interannual variation and bottom-up nitrogen enrichment for plant-pollinator networks. Oikos. 118(12): 1816-1829.
Burton, P.J.; Burton, C.M. 2002. Promoting genetic diversity in the production of large quantities of native plant seed. Ecological Restoration. 20(2): 117-123.
Buss, J.; Stratechuk, K.; Pinno, B.D. 2018. Growth and competition among understory plants varies with reclamation soil and fertilization. Ecological Processes. 7(1): 1-8.
Callow, J.M.; Kantrud, H.A.; Higgins, K.H. 1992. First flowering dates and flowering periods of prairie plants at Woodworth, North Dakota. Prairie Naturalist. 24(2): 57-64.
CaraDonna, P.J. 2016. Temporal ecology of a subalpine ecosystem: Plant communities, plant-pollinator interactions, and climate change. Tucson, AZ: University of Arizona. Dissertation. 123 p.
Carleton, W.M. 1966. Food habits of two sympatric Colorado sciurids. Journal of Mammalogy. 47(1): 91-103.
Carlson, S.R.; Whitford, W.G. 1991. Ant mound influence on vegetation and soils in a semiarid mountain ecosystem. The American Midland Naturalist. 126(1): 125-139.
Chávez, V.; Macdonald, S.E. 2010. The influence of canopy patch mosaics on understory plant community composition in boreal mixedwood forest. Forest Ecology and Management. 259(6): 1067-1075.
Chesnut, V.K. 1902. Plants used by the Indians of Mendocino County, California. Contributions from the United States National Herbarium. 7(3): 295-422.
Clements, D.R.; Krannitz, P.G.; Gillespie, S.M. 2007. Seed bank responses to grazing history by invasive and native plant species in a semi-desert shrub-steppe environment. Northwest Science. 81(1): 37-49.
Collins, W.B.; Urness, P.J. 1983. Feeding behavior and habitat selection of mule deer and elk on northern Utah summer range. The Journal of Wildlife Management. 47(3): 646-663.
Consortium of Pacific Northwest Herbaria [CPNWH]. 2020. Seattle, WA: University of Washington Herbarium, Burke Museum of Natural History and Culture. http://www.pnwherbaria.org/index.php
Cook, S.F., Jr. 1959. The effects of fire on a population of small rodents. Ecology. 40(1): 102-108.
Cork, S.J.; Kenagy, G.J. 1989. Nutritional value of hypogeous fungus for a forest-dwelling ground squirrel. Ecology. 70(3): 577-586.
Courtemanch, A.B.; Kauffman, M.J. 2008. Recruitment, diet composition, and time-budgets of bighorn sheep (Ovis canadensis) in the Teton Range. University of Wyoming National Park Service Research Center Annual Report. 31(12): 73-79.
Craighead, J.J.; Craighead, F.C. Jr.; Davis, R.J. 1963. A field guide to Rocky Mountain wildflowers from northern Arizona and New Mexico to British Columbia. Boston, MA: Houghton Mifflin Company. 277 p.
Crawford, J.A.; Wahren, C.H.A.; Kyle, S.; Moir, W.H. 2001. Responses of exotic plant species to fires in Pinus ponderosa forests in northern Arizona. Journal of Vegetation Science. 12(2): 261-268.
Currah, R.; Smreciu, A.; Van Dyk, M. 1983. Prairie wildflowers: An illustrated manual of species suitable for cultivation and grassland restoration. Edmonton, AB: University of Alberta, Friends of the Devonian Botanic Garden. 290 p.
del Moral, R. 1982. Control of vegetation on contrasting substrates: Herb patterns on serpentine and sandstone. American Journal of Botany. 69(2): 227-238.
del Moral, R. 1984. The impact of the Olympic marmot on subalpine vegetation structure. American Journal of Botany. 71(9): 1228-1236.
Dunnell, K.L.; Travers, S.E. 2011. Shifts in the flowering phenology of the northern Great Plains: Patterns over 100 years. American Journal of Botany. 98(6): 935-945.
Eldredge, E.; Novak-Echenique, P.; Heater, T.; Mulder, A.; Jasmine, J. 2013. Plants for pollinator habitat in Nevada. Tech. Note NV 57. Reno, NV: U.S. Department of Agriculture, Natural Resources Conservation Service. 65 p.
Ellers, J.; Boggs, C.L. 2002. The evolution of wing color in Colias butterflies: Heritability, sex linkage, and population divergence. Evolution. 56(4): 836-840.
Ellison, L. 1954. Subalpine vegetation of the Wasatch Plateau, Utah. Ecological Monographs. 24(2): 89-184.
Emmingham, W.H. 1972. Conifer growth and plant distribution under different light environments in the Siskiyou Mountains of southwestern Oregon. Corvallis, OR: Oregon State University. Thesis. 50 p.
Endo, Y.; Choi, B.H.; Ohashi, H.; Delgado-Salinas, A. 2008. Phylogenetic relationships of New World Vicia (Leguminosae) inferred from nrDNA internal transcribed spacer sequences and floral characters. Systematic Botany. 33(2): 356-363.
Endo, Y.; Ohashi, H. 1997. Cladistic analysis of phylogenetic relationships among tribes Cicereae, Trifolieae, and Vicieae (Leguminosae). American Journal of Botany. 84(4): 523-529.
Erickson, W.R. 2002. Environmental relationships of native Garry oak (Quercus garryana) communities at their northern margin. In: Standiford, R.B.; McCreary, D.; Purcell, K.L., tech. coords. Proceedings of the 5th symposium on oak woodlands: Oaks in California’s changing landscape; 2001 October 22-25; San Diego, CA. Gen. Tech. Rep. PSW-GTR-184. Albany, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station: 179-190.
European Native Seed Conservation Network [ENSCONET]. 2009. ENSCONET seed collecting manual for wild species. Edition 1: 32 p.
Farr, D.F.; Rossman, A.Y. 2017. Fungal databases, U.S. National Fungus Collections. U.S. Department of Agriculture, Agricultural Research Service. https://nt.ars-grin.gov/fungaldatabases/.
Fonda, R.W.; Bernardi, J.A. 1976. Vegetation of Sucia Island in Puget Sound, Washington. Bulletin of the Torrey Botanical Club. 103(3): 99-109.
Forrest, J.; Inouye, D.W.; Thomson, J.D. 2010. Flowering phenology in subalpine meadows: Does climate variation influence community co-flowering patterns? Ecology. 91(2): 431-440.
Franklin, J.F.; Dyrness, C.T. 1973. Natural vegetation of Oregon and Washington. Gen. Tech. Rep. PNW-8. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 452 p.
Gibbens, R. 1954. Root and top development of five native Kansas legumes during first season of growth. Transactions of the Kansas Academy of Science. 57(1): 23-40.
Gilligan, T. 2019. Tortricid.net. Tortricidae resources on the web, version 2.0. Available: http://www.tortricidae.com/.
Gould, K.; Wood, S.; Smreciu, A. 2013. Vicia americana: Peavine, wild pea, American vetch, wild vetch. Alberta, Canada. Available: https://era.library.ualberta.ca/ [Accessed 2021 February 15].
Graham, P.H. 2005. Practices and issues in the inoculation of prairie legumes used in revegetation and restoration. Ecological Restoration. 23(3): 187-195.
Griswold, S.M. 1936. Effect of alternate moistening and drying on germination of seeds of western range plants. Botanical Gazette. 98(2): 243-269.
Gross, D.V.; Romo, J.T. 2010. Temporal changes in species composition in fescue prairie: Relationships with burning history, time of burning, and environmental conditions. Plant Ecology. 208(1): 137-153.
Gunn, C.R. 1970. Seeds of the tribe Vicieae (Leguminosae) in North American agriculture. Proceedings of the Association of Official Seed Analysts. 60: 48-70.
Halpern, C.B. 1989. Early successional patterns of forest species: Interactions of life history traits and disturbance. Ecology. 70(3): 704-720.
Harder, L.D.; Cruzan, M.B. 1990. An evaluation of the physiological and evolutionary influences of inflorescence size and flower depth on nectar production. Functional Ecology. 4(4): 559-572.
Harte, J.; Shaw, R. 1995. Shifting dominance within a montane vegetation community: Results of a climate-warming experiment. Science. 267(5199): 876-880.
Hassell, W.; Beavers, W.R.; Ouellette, S.; Mitchell, T. 1998. Seeding rate statistics for native and introduced species. Plant Materials Technical Note 21. Portland, OR: U.S. Department of Agriculture, Natural Resources Conservation Service. 25 p.
Heidinga, L.; Wilson, S.D. 2002. The impact of an invading alien grass (Agropyron cristatum) on species turnover in native prairie. Diversity and Distributions. 8(5): 249-258.
Hermann, F. 1966. Notes on western range forbs: Cruciferae through Compositae. Agric. Handb. 293. Washington, DC: U.S. Department of Agriculture, Forest Service. 365 p.
Hickman, J.C., ed. 1993. The Jepson manual: Higher plants of California. Berkeley, CA: University of California Press. 1400 p.
Hitchcock, C.L.; Cronquist, A. 2018. Flora of the Pacific Northwest: An illustrated manual. Second Ed. Giblin, D.E.; Legler, B.S.; Zika, P.F.; Olmstead, R.G., eds. Seattle, WA: University of Washington Press. 882 p.
Holcroft, A.C.; Herrero, S. 1991. Black bear, Ursus americanus, food habits in southwestern Alberta. The Canadian Field-Naturalist. 105(3): 335-345.
Hughes, J.B. 2000. The scale of resource specialization and the distribution and abundance of Lycaenid butterflies. Oecologia. 123(3): 375-383.
Inouye, D.W. 1980. The effect of proboscis and corolla tube lengths on patterns and rates of flower visitation by bumblebees. Oecologia. 45(2): 197-201.
ITIS Database. 2022. Integrated Taxonomic Information System. Available: http://www.itis.gov/index.html
James, D.G.; Nunnallee, D. 2011. Life histories of Cascadia butterflies. Corvallis, OR: Oregon State University Press. 447 p.
Johnson, W.C.; Burgess, R.L.; Keammerer, W.R. 1976. Forest overstory vegetation and environment on the Missouri River Floodplain in North Dakota. Ecological Monographs. 46(1): 59-84.
Kasworm, W.F.; Irby, L.R.; Ihsle Pac, H.B. 1984. Diets of ungulates using winter ranges in northcentral Montana. Journal of Range Management. 37(1): 67-71.
Kayes, L.J. 2008. Early-successional vegetation dynamics and microsite preferences following post-fire forest restoration in southwestern Oregon. Corvallis, OR: Oregon State University. Dissertation. 184 p.
Keeler, A.M.; Rafferty, N.E. 2018. Facilitation of range expansion of legumes Lathyrus lanszwertii var. leucanthus and Vicia americana by the microbial symbionts of Lupinus argenteus. In: Ecological Society of America annual meeting; 2018 August 5-10; New Orleans, LA. Abstract. 1 p.
Keeler, A.M.; Rose-Person, A.; Rafferty, N.E. 2021. From the ground up: Building predictions for how climate change will affect belowground mutualisms, floral traits, and bee behavior. Climate Change Ecology. 1: 100013.
Kenicer, G.; Norton, S. 2008. 635. Vicia americana: Leguminosae. Curtis’s Botanical Magazine. 25(4): 342-349.
Kirk, S.; Belt, S. 2010. Plant fact sheet for American vetch (Vicia americana). Beltsville, MD: U.S. Department of Agriculture, Norman A. Berg National Plant Material Center. 2 p.
Kunzler, L.M.; Harper, K.T.; Kunzler, D.B. 1981. Compositional similarity within the oakbrush type in central and northern Utah. The Great Basin Naturalist. 41(1): 147-153.
Langenheim, J.H. 1962. Vegetation and environmental patterns in the Crested Butte Area, Gunnison County, Colorado. Ecological Monographs. 32(3): 249-285.
Larson, M.; Moir, W.H. 1987. Forest and woodland habitat types (plant associations) of northern New Mexico and northern Arizona. 2nd ed. Albuquerque, NM: U.S. Department of Agriculture, Forest Service, Southwestern Region. 90 p.
LeCount, A. 1970. Fall food preferences of blue grouse in the White Mountains of Arizona. Tucson, AZ: University of Arizona. Thesis. 45 p.
LeFebvre, J. 2017. Release brochure: Stucky Ridge Germplasm silverleaf phacelia (Phacelia hastata). Bridger, MT. U.S. Department of Agriculture, Natural Resources Conservation Service. 2 p.
Letts, B.; Lamb, E.G.; Mischkolz, J.M.; Romo, J.T. 2015. Litter accumulation drives grassland plant community composition and functional diversity via leaf traits. Plant Ecology. 216(3): 357-370.
Looman, J. 1969. The fescue grasslands of western Canada. Vegetatio. 19(1/6): 128-145.
Louda, S.M.; Dixon, P.M.; Huntly, N. 1987. Herbivory in sun versus shade at a natural meadow-woodland ecotone in the Rocky Mountains USA. Vegetatio. 72(3): 141-149.
Love, S.L.; Akins, C.J. 2020. Sixth summary of the native seed germination studies of Norman C Deno: Species with names beginning with letters R through Z. Native Plants Journal. 21(2): 150-187.
Luna, T.; Mousseaux, M.R.; Dumroese, R.K. 2018. Common native forbs of the northern Great Basin important for greater sage-grouse. Gen. Tech. Rep. RMRS-GTR-387. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station; Portland, OR: U.S. Department of the Interior, Bureau of Land Management, Oregon-Washington Region. 76 p.
Mackenzie, D.D.; Naeth, M.A. 2017. Effect of plant-derived smoke water and potassium nitrate on germination of understory boreal forest plants. Canadian Journal of Forest Research. 49(12): 1540-1547.
Mackie, R.J. 1970. Range ecology and relations of mule deer, elk, and cattle in the Missouri River Breaks, Montana. Wildlife Monographs. 20: 3-79.
Martin, A.C.; Zim, H.S.; Nelson, A.L. 1951. American wildlife and plants: A guide to wildlife food habits. New York, NY: Dover Publications. 500 p.
McInnis, M.L.; Vavra, M.; Krueger, W.C. 1983. A comparison of four methods used to determine the diets of large herbivores. Journal of Range Management. 36(3): 302-306.
McLean, A. 1969. Fire resistance of forest species as influenced by root systems. Journal of Range Management. 22(2): 120-122.
Merrill, E.H.; Henry F.M.; Peek, J.M. 1980. Effects of a fall wildfire on herbaceous vegetation on xeric sites in the Selway-Bitterroot Wilderness, Idaho. Journal of Range Management. 33(5): 363-367.
Merrill, T.; Mattson, D.J.; Wright, R.G.; Quigley, H.B. 1999. Defining landscapes suitable for restoration of grizzly bears Ursus arctos in Idaho. Biological Conservation. 87(2): 231-248.
Moerman, D. 2003. Native American ethnobotany: A database of foods, drugs, dyes, and fibers of Native American peoples, derived from plants. Dearborn, MI: University of Michigan. http://naeb.brit.org
Morgan, M.D. 1969. Ecology of aspen in Gunnison County, Colorado. The American Midland Naturalist. 82(1): 204-228.
Mueggler, W.F.; Stewart, W.L. 1980. Grassland and shrubland habitat types of western Montana. Gen. Tech. Rep. INT-66. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 154 p.
Munz, P.A.; Keck, D.D. 1973. A California flora and supplement. Berkeley, CA: University of California Press. 1905 p.
Nagel, H.G. 1995. Vegetative changes during 17 years of succession on Willa Cather Prairie in Nebraska. In: Hartnett, D.C., ed. Prairie biodiversity: Proceedings, 14th North American prairie conference; 1994 July 12-16; Manhattan, KS. Manhattan, KS: Kansas State University: 25-30.
Nielsen, M.G.; Watt, W.B. 1998. Behavioural fitness component effects of the alba polymorphism of Colias (Lepidoptera, Pieridae): Resource and time budget analysis. Functional Ecology. 12(1): 149-158.
Ogle, D.; Tilley, D.; Cane, J.; St. John, L.; Fullen, K.; Stannard, M.; Pavek, P. 2017. Plants for pollinators in the Intermountain West, revised. Tech. Note 2A. Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 44 p.
Ogle, D.; Tilley, D.; St. John, L.; Stannard, M.; Holzworth, L. 2014. Conservation plant species for the Intermountain West. Plant Materials Tech. Note 24. Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 57 p.
Omernik, J.M. 1987. Ecoregions of the conterminous United States. Map (scale 1:7,500,000). Annals of the Association of American Geographers. 77(1): 118-125.
Pengelly, C.J.; Cartar, R.V. 2011. Effect of boreal forest logging on nectar production of four understory herbs. Forest Ecology and Management. 261(11): 2068-2074.
Peterson, J.G. 1969. The food habits and summer distribution of juvenile sage grouse in central Montana. Bozeman, MT: Montana State University. Thesis. 39 p.
Peterson, J.G. 1970. The food habits and summer distribution of juvenile sage grouse in central Montana. Journal of Wildlife Management. 34(1): 147-155.
Plant Conservation Alliance [PCA]. 2015. National seed strategy for rehabilitation and restoration 2015-2020. Washington, DC: U.S. Department of the Interior, Bureau of Land Management. 52 p.
Pleasants, J.M. 1980. Competition for bumblebee pollinators in Rocky Mountain plant communities. Ecology. 61(6): 1446-1459.
Pokorny, M.L.; Sheley, R.L.; Svejcar, T.J.; Engel, R.E. 2004. Plant species diversity in a grassland plant community: Evidence for forbs as a critical management consideration. Western North American Naturalist. 64(2): 219-230.
Quinnild, C.L.; Cosby, H.E. 1958. Relicts of climax vegetation on two mesas in western North Dakota. Ecology 39(1): 29-32.
Rantala-Sykes, B.; Campbell, D. 2018. Can fertilizers increase the seed yield of two native herb species in the subarctic? Implications for wild seed collection. Ecological Restoration. 36(3): 169-171.
Reinhart, K.O.; Lekberg, Y.; Klironomos, J.; Maherali, H. 2017. Does responsiveness to arbuscular mycorrhizal fungi depend on plant invasive status? Ecology and Evolution. 7(16): 6482-6492.
Richardson, B.; Kilkenny, F.; St. Clair, B.; Stevenson-Molnar, N. 2020. Climate Smart Restoration Tool. https://climaterestorationtool.org/csrt/
Riegel, G.M.; Svejcar, T.J.; Busse, M.D. 2002. Does the presence of Wyethia mollis affect growth of Pinus jeffreyi seedlings? Western North American Naturalist. 62(2): 141-150.
Riegel, G.M.; Thornburgh, D.A.; Sawyer, J.O. 1990. Forest habitat types of the South Warner Mountains, Modoc County, California. Madrono. 37(2): 88-112.
Ross, R.L.; Hunter, H.E. 1976. Climax vegetation of Montana: Based on soils and climate. Bozeman, MT: U.S. Department of Agriculture, Soil Conservation Service. 64 p.
Rowe, J.S. 1956. Uses of undergrowth plant species in forestry. Ecology 37(3): 461-473.
Russell, W.B. 1980. The vascular flora and natural vegetation of abandoned coal mined land, Rocky Mountain foothills, Alberta. Edmonton, Alberta: University of Alberta. Thesis. 96 p.
Ruyle, G.B.; Bowns, J.E. 1985. Forage use by cattle and sheep grazing separately and together on summer range in southwestern Utah. Journal of Range Management. 38(4): 299-302.
Schladweiler, B.K.; Vance, G.F.; Legg, D.E.; Munn, L.C.; Haroian, R. 2005. Topsoil depth effects on reclaimed coal mine and native area vegetation in northeastern Wyoming. Rangeland Ecology and Management. 58(2): 167-176.
SEINet – Regional Networks of North American Herbaria Steering Committee [SEINet]. 2020. SEINet Regional Networks of North American Herbaria. https://Symbiota.org/docs/seinet
Shantz, H.L. 1906. A study of the vegetation of the Mesa Region east of Pike’s Peak: The Bouteloua formation. II. Development of the formation. Botanical Gazette. 42(3): 179-207.
Shiflet, T.N. 1994. Rangeland cover types of the United States. Denver, CO: Society for Range Management. 152 p.
Sieg, C.H.; Uresk, D.W.; Hansen, R.M. 1983. Plant-soil relationships on bentonite mine spoils and sagebrush-grassland in the northern High Plains. Journal of Range Management. 36(3): 289-294.
Sinkins, P.A.; Otfinowski, R. 2012. Invasion or retreat? The fate of exotic invaders on the northern prairies, 40 years after cattle grazing. Plant Ecology. 213(8): 1251-1262.
Smith, D.W. 1969. A preliminary classification and characterization of big sagebrush, Artemisia tridentata Nutt., communities in central Montana. Bozeman, Montana: Montana State University. Thesis. 68 p.
Smith, J.G. 1949. Deer forage observations in Utah. The Journal of Wildlife Management. 13(3): 314-315.
Smreciu, A.; Gould, K. 2015. Field emergence of native boreal forest species on reclaimed sites in northeastern Alberta. Native Plants Journal. 16(3): 204-226.
Society for Ecological Restoration; International Network for Seed Based Restoration; Royal Botanic Gardens [SER, INSR, RBGK]. 2023. Seed Information Database (SID). Available from: https://ser-sid.org/
Soper, J.D. 1941. History, range, and home life of the northern Bison. Ecological Monographs. 11(4): 348-412.
Spellenberg, R. 2001. National Audubon Society field guide to North American wildflowers: Western region, revised edition. New York, NY: Alfred A. Knopf, Inc. 862 p.
Stanton, M.L. 1982. Searching in a patchy environment: Foodplant selection by Colis p. eriphyle butterflies. Ecology. 63(3): 839-853.
Stark, K.E.; Andre, A.; Bradfield, G.E. 2006. Soil seed banks and plant community assembly following disturbance by fire and logging in interior Douglas-fir forests of south-central British Columbia. Canadian Journal of Botany. 84(10): 1548-1560.
Stevens, O.A. 1957. Weights of seeds and numbers per plant. Weeds. 5(1): 46-55.
Sugihara, N.G.; Reed, L.J.; Lenihan, J.M. 1987. Vegetation of the Bald Hills oak woodlands, Redwood National Park, California. Madrono. 34(3): 193-208.
Taliga, C. 2011. Plant suitability and seeding rates for conservation plantings in Colorado. Tech. Note 59. Denver, CO: U.S. Department of Agriculture, State of Colorado, Natural Resources Conservation Service. 21 p.
Thacker, E.T.; Springer, T.L. 2016. Preference of pen-reared northern bobwhite among native plant seeds of the sand sagebrush-mixed prairie. The Southwestern Naturalist. 61(4): 307-311.
Thill, R.E.; Ffolliott, P.F.; Patton, D.R. 1983. Deer and elk forage production in Arizona mixed conifer forests. Res. Pap. RM-248. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 13 p.
Thilmony, B.M.; Rodney, G.L. 2017. Effect of aminocyclopyrachlor on native prairie species in the northern Great Plains. Invasive Plant Science and Management. 10(2): 201-209.
Thomas, R.C.; Schultz, C.B. 2016. Resource selection in an endangered butterfly: Females select native nectar species. The Journal of Wildlife Management. 80(1): 171-180.
Uresk, D.W.; Severson, K.E. 1998. Response of understory species to changes in ponderosa pine stocking levels in the Black Hills. The Great Basin Naturalist. 58(4): 312-327.
USDA Forest Service [USDA FS] 1937. Range plant handbook. Washington, DC: U.S. Department of Agriculture, Forest Service. 816 p.
USDA Forest Service, Bend Seed Extractory [USDA FS BSE]. 2017. Nursery Management Information System Version 4.1.11. Local Source Report 34-Source Received. Bend, OR: U.S. Department of Agriculture, Forest Service, Bend Seed Extractory.
USDA Forest Service, Great Basin Native Plant Project [USFS GBNPP]. 2014. Seed weight table calculations made in-house. Report on file. Boise, ID: U.S. Department of Agriculture, Forest Service, Boise Aquatic Sciences Laboratory. Available: https://www.fs.fed.us/rm/boise/research/shrub/Links/Seedweights.pdf
USDA Forest Service, Western Wildland Environmental Threat Assessment Center [USFS WWETAC]. 2017. TRM Seed Zone Applications. Prineville, OR: U.S. Department of Agriculture, Forest Service, Western Wildland Environmental Threat Assessment Center. https://www.fs.fed.us/wwetac/threat-map/TRMSeedZoneMapper.php
USDA Natural Resources Conservation Service [USDA NRCS]. 2021. The PLANTS Database. Greensboro, NC: U.S. Department of Agriculture, Natural Resources Conservation Service, National Plant Data Team. https://plants.usda.gov/java
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2016. Bureau of Land Management technical protocol for the collection, study, and conservation of seeds from native plant species for Seeds of Success. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program. 37 p.
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2020. Seeds of Success collection data. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program.
USDI Environmental Protection Agency [USDI EPA]. 2017. Ecoregions. Washington, DC: U.S. Department of the Interior, Environmental Protection Agency. https://www.epa.gov/eco-research/ecoregions
USDI Geological Survey [USDI USGS]. 2020. Biodiversity Information Serving Our Nation (BISON). U.S. Geological Survey. https://www.gbif.us/#home
Utah Crop Improvement Association [UCIA]. 2015. How to be a seed connoisseur. Logan, UT: UCIA, Utah Department of Agriculture and Food, Utah State University and Utah State Seed Laboratory. 16 p.
Vaughan, T.A. 1974. Resource allocation in some sympatric, subalpine rodents. Journal of Mammalogy. 55(4): 764-795.
Vestal, P.A. 1952. Ethnobotany of the Ramah Navaho. Reports of the Ramah Project: No. 4. Papers of the Peabody Museum of American Archeology and Ethnology. Cambridge, MA: Harvard University. 40(4): 1-94.
Walcheck, K.C. 1970. Nesting bird ecology of four plant communities in the Missouri River Breaks, Montana. The Wilson Bulletin. 82(4): 370-382.
Walker, S.C.; Anderson, V.J.; Fugal, R.A. 2015. Big game and cattle influence on aspen community regeneration following prescribed fire. Rangeland Ecology and Management. 68(4): 354-358.
Wang, G.G.; Kemball, K.J. 2005. Effects of fire severity on early development of understory vegetation. Canadian Journal of Forest Research. 35(2): 254-262.
Wasser, C.H. 1982. Ecology and culture of selected species useful in revegetating disturbed lands in the West. FWS/OBS-82/56. Washington, D.C.: U.S. Department of the Interior, Fish and Wildlife Service, Office of Biological Services, Western Energy and Land Use Team. 347 p.
Watt, W.B.; Hoch, P.C.; Mills, S.G. 1974. Nectar resource use by Colias butterflies. Chemical and visual aspects. Oecologia. 14(4): 353-374.
Weaver, J.E.; Stoddart, L.A.; Wm, N. 1935. Response of the prairie to the great drought of 1934. Ecology. 16(4): 612-629.
Weaver, T.; Gustafson, D.; Lichthardt, J. 1993. Plants colonizing disturbed areas in fifteen Rocky Mountain environments: Weeds and reclamation candidates. University of Wyoming National Park Service Research Center annual report. 17(4): 1-9.
Weigand, J.P. 1980. Ecology of the Hungarian partridge in north-central Montana. Wildlife Monographs. (74): 3-106.
Welch, B.L. 2004. Nutritive principles in restoration and management. In: Monsen, S.B.; Stevens, R.; Shaw, N.L., comps. Restoring western ranges and wildlands, vol. 1. Gen. Tech. Rep. RMRS-GTR-136-vol-1. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 175-186.
Welsh, S.L.; Atwood, N.D.; Goodrich, S.; Higgins, L.C., eds. 2015. A Utah Flora. Fifth Edition, revised. Provo, UT: Brigham Young University. 990 p.
West, N.E.; Hassan, M.A. 1985. Recovery of sagebrush-grass vegetation following wildfire. Journal of Range Management. 38(2): 131-134.
Wiens, D. 1984. Ovule survivorship, brood size, life history, breeding systems, and reproductive success in plants. Oecologia. 64(1): 47-53.
Wilson, J.L.; Ayres, D.R.; Steinmaus, S.; Baad, M. 2009. Vegetation and flora of a biodiversity hotspot: Pine Hill, El Dorado County, California USA. Madrono. 56(4): 246-278.
Winslow, S.R.; Clause, K.J.; Hybner, R.; Jacobs, J. n.d. Evaluating seeding techniques and native plant establishment in the Pinedale Anticline, Wyoming. Bridger, MT: U.S. Department of Agriculture, Natural Resources Conservation Service, Plant Materials Center. 25 p.
Winslow, S.R.; Clause, K.J.; Jacobs, J.S.; Hybner, R.M. 2009. Revegetation trials in the Pinedale anticline project area. In: Barnhisel, R.I., ed. National Meeting of the American Society of Mining and Reclamation; 2009 May 30-June 5; Billings, MT. Lexington, KY: American Society of Mining and Reclamation. 35 p.
Woolley, S.B., comp. 1936. Root systems of important range plants of the Boise River watershed: A catalogue of species excavated by Liter E. Spence [collaborator]. Unpublished paper on file at: U.S. Department of Agriculture, Forest Service, Intermountain Fire Sciences Lab, Missoula, MT. p. 59.
Young, S.A.; Schrumpf, B.; Amberson, E. 2003. The Association of Official Seed Certifying Agencies (AOSCA) native plant connection. Moline, IL: AOSCA. 9 p. Available: https://seedcert.oregonstate.edu/sites/seedcert.oregonstate.edu/files/pdfs/aoscanativeplantbrochure.pdf
Zimmerman, G.T.; Neuenschwander, L.F. 1984. Livestock grazing influences on community structure, fire intensity, and fire frequency within the Douglas-fir/ninebark habitat type. Journal of Range Management 37(2): 104-110.
How to Cite
Gucker, Corey L.; Shaw, Nancy L. 2022. American vetch (Vicia americana). In: Gucker, C.L.; Shaw, N.L., eds. Western forbs: Biology, ecology, and use in restoration. Reno, NV: Great Basin Fire Science Exchange. Online: https://westernforbs.org/species/american-vetch-vicia-americana/