Authorship
Gucker, Corey; Shaw, Nancy
Publication Date
November 2021
Nomenclature
Common woolly sunflower (Eriophyllum lanatum [Pursh] Forbes) belongs to the Heliantheae tribe of the Asteraceae family (Johnson and Mooring 2006).
Family
Asteraceae – Aster family
Genus
Eriophyllum
Species
lanatum
NRCS Plant Code
ERLA6 (USDA NRCS 2021).
Subtaxa
The Flora of North America (Johnson and Mooring 2006) recognizes 10 common woolly sunflower varieties: achillioides (D.C.) Jeps. (alternate spellings include: achilleoides and achillioides), arachnoideum (Fisch. & Ave-Lall.) Jeps., croceum (Greene) Jeps., grandiflorum (A. Gray) Jeps., hallii (Constance), integrifolium (Hook.) Smiley, lanatum (Pursh) Forbes, lanceolatum (Howell) Jeps., leucophyllum (D.C.) W.R. Carter, and obovatum (Greene) H.M. Hall.
Synonyms
Variety achillioides: E. l. var. aphanactis J.T. Howell, Bahia achilleoides (de Candolle)
Variety arachnoideum: B. arachnoidea (Fisch. & Ave–Lall.)
Variety croceum: E. croceum (Greene);
Variety grandiflorum: B. lanata var. grandiflora (Gray)
Variety lanatum: E. l. var. typicum Constance
Variety lanceolatum: E. lanceolatum (Howell)
Variety leucophyllum: B. leucophylla (de Candolle)
Variety integrifolium: E. integrifolium (Hook.) Greene, Trichophyllum integrifolium (Hook.), E. lanatum var. cunteaum (Kellogg) Jeps., E. l. var. monoense (Rydb.) Jeps.
Variety obovatum: E. obovatum (Greene) (Johnson and Mooring 2006; ITIS 2021)
Common Names
Common woolly sunflower, Fort Tejon woolly sunflower, Oregon sunshine, Pursh’s woollyleaf, woolly daisy, woolly eriophyllum, woolly yellowdaisy, yarrow-leaved eriophyllum (Craighead et al. 1963; Taylor 1992; Hickman 1993; Lambert 2005; Pavek 2011; Pavek et al. 2012; LBJWC 2016; Welsh et al. 2016).
Chromosome Number
Chromosome numbers are: 2n = 16 for varieties hallii and obovatum, 2n = 16, 32 for varieties achillioides, arachnoideum, croceum, lanatum, leucophyllum, and lanceolatum, and 2n = 16, 32, 48, 64 for varieties integrifolium and grandiflorum (Johnson and Mooring 2006).
Hybridization
Common woolly sunflower is a polyploid complex of intergrading varieties. Several varieties also hybridize with golden-yarrow (E. confertiflorum) (Mooring 2001; Johnson and Mooring 2006). Morphologically intermediate polyploid populations occur where variety ranges overlap (Johnson and Mooring 2006), primarily in northern California, western Oregon, and near the Columbia River, where varieties achillioides, arachnoideum, grandiflorum, lanatum, lanceolatum, or leucophyllum connect. In Oregon’s Klamath region, “it seems quite impossible to assign many specimens definitely to any single [varietal] category” (Constance 1937 cited in Johnson and Mooring [2006]).
In studies of more than 400 common woolly sunflower plants representing more than 270 populations, most populations contained only a single ploidy level (Mooring 1975, 2001). A little more than 70% of populations were diploid, 22% tetraploid, 3% hexaploid, and 3% octoploid. Polyploid populations were four times more common among plants judged to be intermediates between varieties and had more restricted geographic distributions than diploid populations (Fig. 1; Mooring 1975, 2001). Polyploidy was not correlated with site characteristics such as geological history, soils, topography, or climate (Carlquist 1956). The primary role of polyploidy has been to stabilize products of hybridization. Studies suggest that polyploidy in the common woolly sunflower complex has 1) supported intervarietal hybridization, 2) prevented or decreased interbreeding between individuals of different varieties in mixed stands, and 3) probably produced new species through hybridization (e.g., San Mateo woolly sunflower [E. latilobum], Jepson’s woolly sunflower [E. jepsonii], and golden–yarrow [E. confertiflorum var. tanacetiflorum] (Mooring 1975, 2001; Johnson and Mooring 2006).
Distribution
Common woolly sunflower occurs from British Columbia south to California, east to Nevada, northwestern Utah, and western Wyoming and Montana (Johnson and Mooring 2006).
Variety integrifolium is the most widely distributed of the common woolly sunflower varieties (Table 1). Varieties obovatum and hallii are uncommon (Mooring 2001). Variety hallii is known from only two populations in Kern and Santa Barbara counties, California (Tibor 2001 cited in Johnson and Mooring [2006]).

Figure 1. Distribution of diploids (2x), tetraploids (4x), hexaploids (6x), and octoploids (8x) in common woolly sunflower (symbols). Range for the species is outlined (dashed line) (from Mooring 2001).
Table 1. Presence of common woolly sunflower varieties by provinces and states in western North America (Johnson and Mooring 2006).
|
Variety |
BC |
WA |
OR |
CA |
NV |
nw UT |
ID |
w WY |
w MT |
|
achillioides |
X |
X |
X |
||||||
|
arachnoideum |
X |
||||||||
|
croceum |
|||||||||
|
grandiflorum |
X |
X |
|||||||
|
hallii |
X |
||||||||
|
integrifolium |
X |
X |
X |
X |
X |
X |
X |
X |
|
|
lanatum |
X |
X |
X |
X |
|||||
|
lanceolatum |
X |
X |
|||||||
|
leucophyllum |
X |
X |
X |
||||||
|
obovatum |
X |
Habitat And Plant Associations
Common woolly sunflower occurs in diverse plant communities from near sea level to alpine elevations (Mooring 1975). In California, it occurs from dry canyons to moist uplands in grasslands, chaparral, oak (Quercus spp.) and juniper (Juniperus spp.) woodlands; mixed evergreen forests (Fig. 2); and alpine fell fields. It is especially common in brush habitats (Munz and Keck 1973; Hickman 1993). In the Walker River drainage in central California and west-central Nevada, common woolly sunflower occurred in nearly all vegetation sampled along an increasing elevation gradient, which included sagebrush (Artemisia spp.)-grasslands, pinyon (Pinus spp.)-juniper woodlands, Jeffrey pine (P. jeffreyi) forests, lodgepole pine (P. contorta) forests, and alpine communities (Lavin 1983).
Common woolly sunflower is widespread in the Great Basin and grows in dry prairies and grasslands to above timberline in alpine sites (Lambert 2005; Blackwell 2006). In Grand Teton National Park and surrounding Bridger-Teton and Targhee National Forests, common woolly sunflower grows in willow (Salix spp.) bottomlands, sagebrush shrublands, montane meadows, mixed–conifer forests, and subalpine habitats (Kesonie and Hartman 2011).
Alpine vegetation. On Glass Mountain in Mono County, California, common woolly sunflower grows on dry windswept pumice slopes and plateaus with or without whitebark pine (P. albicaulis) overstories (Horner 2001). In the Teton Mountains of Wyoming, it is common in subalpine vegetation and occurs on neoglacial deposits in the treeless alpine zone above 9,500 ft (2,900 m) in elevation (Spence and Shaw 1981).
Forests. Constancy of common woolly sunflower was high (up to 83%) in Jeffrey pine forests in southwestern Oregon (Atzet et al. 1996). It was considered a diagnostic forb in ponderosa pine/antelope bitterbrush/Idaho fescue (P. ponderosa/Purshia tridentata/Festuca idahoensis) forests at sites below 5,700 ft (1,700 m) in elevation in south-central Oregon’s pumice region (Dyrness and Youngberg 1966).
Woodlands. Common woolly sunflower is a common forb in central Oregon’s juniper zone (Fig. 3). This zone includes western juniper (J. occidentalis)-dominated woodlands on mostly sandy loam soils in Crook, Deschutes, and Jefferson counties (Driscoll 1964). It is also a typical associate in the western juniper/big sagebrush (Artemisia tridentata)/bluebunch wheatgrass (Pseudoroegneria spicata) rangeland cover type (Shiflet 1994). In southwestern Oregon, common woolly sunflower is common in the understory of Oregon white oak-alderleaf mountain mahogany (Q. garryana–Cercocarpus montanus) communities, which are restricted to ridges and rocky rock outcrops (Riegel and Franklin 1992).

Figure 2. Common woolly sunflower growing in an opening in a mixed–conifer forest in California. Photo: BLM ID230 SOS.

Figure 3. Common woolly sunflower growing in a juniper–sagebrush community in Oregon. Photo: BLM OR050 SOS.
Shrublands. Common woolly sunflower often grows in sagebrush and chaparral communities (Taylor 1992; Stuart et al. 1996). It is also a conspicuous forb in curl-leaf mountain mahogany/arrowleaf balsamroot (C. ledifolius/Balsamorhiza sagittata) communities in the south Warner Mountains of northeastern California, occupying southeastern slopes at 5,705 to 7,923 ft (1,739–2,415 m) in elevation (Riegel et al. 1990). Its constancy was high (90%) in the buckbrush (Ceanothus cuneatus)/common woolly sunflower community type in Castle Crags State Park in Shasta County California. This vegetation type occupies predominantly steep southern slopes (47%) at 3,000 ft (920 m) in elevation (Stuart et al. 1996).
Grasslands. Common woolly sunflower occurs in perennial bunchgrass communities throughout its range (Fig. 4; Franklin and Dyrness 1973; Johnson and Swanson 2005) and in serpentine annual grasslands in California (Rodríguez-Rojo et al. 2001). On Mount Rainier in Washington, common woolly sunflower grows in the driest subalpine meadows dominated by greenleaf fescue (F. viridula) and Cascade aster (Eucephalus ledophyllus var. ledophyllus) (Franklin and Dyrness 1973). In Washington’s Olympic Mountains it grows in dry, Idaho fescue-dominated vegetation on steep south slopes (Kuramoto and Bliss 1970). Cover of common woolly sunflower can be as high as 20% in Idaho fescue-bluebunch wheatgrass-turpentine wavewing (Pteryxia terebinthina var. foeniculacea) grasslands in the Blue and Wallowa Mountains in Oregon. This vegetation type occurs at 6,040 to 8,100 ft (1,840–2,470 m) in elevation on moderately steep (mean 36%) southwestern slopes (Johnson and Swanson 2005).

Figure 4. Common woolly sunflower growing in a grassland at King Hill, Elmore County, Idaho. Photo taken in June: BLM ID230 SOS.
Elevation
The elevation range for common woolly sunflower is 0 to 11,500 ft (3,500 m) (Table 2; Munz and Keck 1973; Mooring 2001; Blackwell 2006).
Table 2. General habitats (Hickman 1993; Johnson and Mooring 2006) and elevation ranges of common woolly sunflower varieties.
|
Variety |
Habitat |
Elevation range (ft) |
|
achillioides |
dry, often rocky sites in chaparral and forests |
100–4,300 (Hickman 1993; Johnson and Mooring 2006) |
|
arachnoideum |
moist places on coastal bluffs and shady banks, and in inland sites with conifers |
0–2,300 (Munz and Keck 1973; Hickman 1993; Johnson and Mooring 2006) |
|
croceum |
usually with conifers |
2,500–6,600 (Munz and Keck 1973; Hickman 1993; Johnson and Mooring 2006) |
|
grandiflorum |
dry, generally rocky area in grasslands and lower montane sites |
100–5,600 (Hickman 1993; Johnson and Mooring 2006) |
|
hallii |
rare in dry woodlands |
3,500–5,000 (Munz and Keck 1973; Hickman 1993; Johnson and Mooring 2006) |
|
lanatum |
moist meadows and dry, rocky coastal or inland sites |
650–3,600 (Johnson and Mooring 2006) |
|
lanceolatum |
dry, rocky sites in oak woodlands and conifer forests |
650–7,200 (Hickman 1993; Johnson and Mooring 2006) |
|
leucophyllum |
dry, rocky sites in oak woodlands and conifer forests |
0–2,600 (Johnson and Mooring 2006), <1,000 in CA (Munz and Keck 1973) |
|
integrifolium |
dry, rocky sites in sagebrush, conifer forests, and alpine fell fields |
4,000–11,500 (Johnson and Mooring 2006), 5,000–7,000 in UT (Welsh et al. 2016) |
|
obovatum |
uncommon in open conifer forests |
4,300–8,200 (Hickman 1993; Johnson and Mooring 2006) |
Soils
Common woolly sunflower is highly drought tolerant and often grows in dry, sandy to rocky soils (Fig. 5) (Craighead et al. 1963; Chambers 1974; Taylor 1992; LBJWC 2016). It is common in the understory of Oregon white oak-alderleaf mountain mahogany communities in southwestern Oregon, where it is restricted to ridges and rocky outcrops in soils with 47% coarse fragments (Riegel and Franklin 1992).

Figure 5. Common woolly sunflower (variety achillioides) growing at a very rocky site in California. Photo taken in June: BLM CA330 SOS.
Common woolly sunflower occupies soils derived from various parent materials. It grows on sandstone cliff escarpments in the Coast Range in Coos County, Oregon (Thompson and Skeese 2005). In Wyoming’s Wind River Range, it occurs in sparsely vegetated badlands on highly eroded clay-rich soils and in dry meadows in relatively well-drained, fine-textured, or colluvial soils on limestone or calcareous outcrops (Fertig et al. 2013). It is also common on serpentine soils in Oregon and California (Walker 1954; Franklin and Dyrness 1973; Atzet et al. 1996). In the Siskiyou Mountains of Oregon and California, common woolly sunflower grows on olivine gabbro and serpentine soils at lower elevations and on diorite soils at higher elevations. Frequency of plants was greatest at the most xeric sites and greater in the open than beneath shrub cover (Whittaker 1960).
Depth. In an evaluation of six common woolly sunflower populations occurring from southeastern Oregon to east-central Washington, populations occurred in soils ranging from 7 in (17 cm) to more than 32 in (80 cm) deep (Hendricks 2016). The buckbrush/common woolly sunflower community type in Castle Crags State Park, California, grows on stony loam, serpentine or greenstone soils that are 20 to 40 in (50–100 cm) deep (Stuart et al. 1996). In grasslands in the Blue and Wallowa mountains of Oregon, where common woolly sunflower can be common, soils are dry, gravelly, sandy loams that overlay bedrock at 21 to 39 in (53–99 cm) deep (Johnson and Swanson 2005). Common woolly sunflower grows in the most xeric of the western juniper zone where soils have an impenetrable caliche layer at 21 in (53 cm) below the soil surface (Driscoll 1964).
Nutrients. Soils with low nutrient levels were reported in the few studies evaluating soil nutrient content where common woolly sunflower occurred. In open Jeffrey pine forests in Siskiyou County, California, common woolly sunflower grew on young serpentinized peridotite soils. These soils were 46 to 56% sand, 29 to 31% silt, and 15 to 25% clay with a pH of 6 to 7, iron levels of 6.2 to 10.5%, calcium/magnesium ratios of less than 0.4, and low productivity (Alexander 1988). When ecological conditions were compared across the natural distribution of redwood (Sequoia sempervirens) forests (Brookings, OR, to Monterey Co, CA), common woolly sunflower was most common at sites within the second lowest moisture and lowest nutrient levels. Average minimum available soil moisture was 9.4% of storage capacity and calcium level averaged 11.7 Ca++Eq/m².30cms (Waring and Major 1964). In the evaluation of six common woolly sunflower populations from southeastern Oregon to east–central Washington, populations occupied sites where soil pH ranged from 5.4 to 7.1. Soils were 18 to 87% sand, 0 to 43% clay, and 11 to 62% silt. Density of common woolly sunflower plants was positively correlated with nitrate and total inorganic nitrogen (r = 0.76) and negatively correlated with silt fraction (r = -0.66) (Hendricks 2016).
Description
Common woolly sunflower is a highly variable species (Figs. 6 and 7) that grows as an annual, biennial, perennial, or subshrub. Variation within the species occurs through intergradation (see Hybridization section) and edaphic conditions that affect the characteristics of plant structures (Mooring 1975, 2001; Johnson and Mooring 2006). In low-elevation, arid habitats, plants are short-lived perennials, biennials, or possibly annuals. In subalpine habitats, plants are longer lived. Subshrub growth forms of up to 3.5 ft (1 m) tall are more common at low elevations, and plants hugging the ground are more common at high elevations (Mooring 1975, 2001; Blackwell 2006). Field observations and garden experiments by Mooring (1975, 2008) revealed very different growth by varieties leucophyllum and achillioides. Variety leucophyllum grows as a long–lived perennial, occurs as clumps, produces massive fibrous roots with abundant root buds, and readily roots from prostrate stems. These plants rarely flower in their first year. Variety achillioides grows as a biennial (or annual) or short-lived perennial, occurs as single individual plants, and produces slight taproots without root buds. These plants often flower in their first year (Mooring 1975, 2008). Varieties are described in more detail in Appendix 1.

Figure 6. Small-stature common woolly sunflower (<6 in tall) growing in Idaho. Photo: BLM ID230 SOS.
Common woolly sunflower plants range from 4 to 40 in (10–100 cm) tall with several to many, simple to few branched, and erect to decumbent stems (Hickman 1993; Johnson and Mooring 2006; Hitchcock and Cronquist 2018). Plants are often taprooted with a branched woody caudex. Herbage is often covered with fine white hairs, appearing grayish-green (Craighead et al. 1963; Munz and Keck 1973; Spellenberg 2001; Pavek et al. 2012; Welsh et al. 2016; Luna et al. 2018). Leaves are usually slender, revolute, or plane, 0.4 to 3 in (1–8 cm) long, entire to two- or three-pinnate with irregular lobes with toothed, serrate, or entire margins (Taylor 1992; Spellenberg 2001; Blackwell 2006; Johnson and Mooring 2006; Welsh et al. 2016).
Flower heads measure 1 to 2.5 in (3–6 cm) across and occur singly or in loosely arrays of five or more at stem ends (Fig. 8; Munz and Keck 1973; Spellenberg 2001; Pavek et al. 2012; Welsh et al. 2016). Flower heads contain 0 to 15 ray florets and 20 to 300 disk florets (Fig. 8; Blackwell 2006; Johnson and Mooring 2006; Hitchcock and Cronquist 2018). Ray florets are pistillate and fertile with 6 to 20 mm long ligules that are shallowly toothed at the tip (Johnson and Mooring 2006). Disk florets are bisexual and fertile. Both floret types are yellow, but ray flowers are often darker toward the base (Taylor 1992; Johnson and Mooring 2006). Peduncles are 1 to 12 in (3–30 cm) tall. Involucres are campanulate to hemispheric with almost equal diameter and height (6–15 mm). Bracts are 5 to 15, distinct or connate at the bases, and prominently ridged on the back (Munz and Keck 1973; Spellenberg 2001; Johnson and Mooring 2006; Welsh et al. 2016; Hitchcock and Cronquist 2018).

Figure 7. Stout common woolly sunflower plant growing in Oregon. Photo: BLM OR050 SOS.

Figure 8. Common woolly sunflower flowers terminal at the ends of stems. Plant growing in Oregon. Photo taken on June 8, 2020: BLM OR134C SOS.
Common woolly sunflower produces cypselae fruits (Johnson and Mooring 2006), hereafter referred to as seeds. Seeds are narrow, linear (2–5 mm), four-sided, gray to black, and tapered at the base. Seeds are glabrous, hairy, or glandular and typically topped with a pappus of 6 to 12 variable chaffy scales (Munz and Keck 1973; Johnson and Mooring 2006; Wall and MacDonald 2009; Welsh et al. 2016).
Reproduction
Common woolly sunflower reproduces by seed. It is considered a prolific seed producer and colonizer of open sites (Pavek 2011), yet a high percentage of seeds can be sterile (Wall and MacDonald 2009) or lost to predation (Mooring 2001; see also Wildlife and Livestock Use section).
In an analysis of six common woolly sunflower populations growing in southeastern Oregon to east-central Washington, plant biomass and reproduction were strongly positively correlated (r = 0.63), but reproduction was not correlated with the site variables analyzed (r ≤ 0.18) (Hendricks 2016). The proportion of plants flowering ranged from 13 to 57%, and a single plant produced 253 flowers, which was twice that of any other individual plant censused in other common woolly sunflower populations. Populations were censused once between March and June 2015. See the Soils section for a discussion on the relationships between plant densities and site variables (Hendricks 2016).
Pollination
Common woolly sunflower produces flowers from April to August depending on variety, elevation, and likely other site conditions (Spellenberg 2001; Blackwell 2006; Johnson and Mooring 2006; Dorn and Dorn 2007) (see Appendix 1). Flowers (Fig. 9) are visited by a variety of beetles, bees, and lepidopterans. Common pollinators include sweat bees (Halictidae), mining bees (Andrenidae), mason bees (Osmia spp.), hover or syrphid flies (Syrphidae), tachinid flies (Tachinidae), and wasps (Mooring 1975; Cane 2011 personal communication, cited in Pavek [2011]; Bartow 2015).
Bagging studies of hundreds of plants from at least 39 populations show that self-incompatibility approaches 99% (Mooring 1975, 2001).
Evaluation of the effects of a nonnative species (hairy cat’s ear [Hypochaeris radicata]) on the pollination of common woolly sunflower revealed that hairy cat’s ear received significantly (P < 0.001) more pollinator visits per inflorescence per hour than common woolly sunflower in all neighborhoods evaluated (Waters et al. 2014). Neighborhoods included: 1) high native and low nonnative floral density, 2) high nonnative and low native floral density, and 3) clipped nonnative neighborhoods originally with a high density of hairy cat’s ear and low native floral density.
The study (Waters et al. 2014) occurred in Puget Trough prairies in western Washington and included nonnative and native species producing similar flowers at a similar time: hairy cat’s ear (nonnative), common woolly sunflower (native), and cutleaf silverpuffs (Microseris laciniata) (native). Common woolly sunflower was visited by solitary bees and syrphid flies but not eusocial bees. Syrphid flies preferentially visited common woolly sunflower in nonnative neighborhoods (P < 0.001). Visitation rates to common woolly sunflower and hairy cat’s ear were higher in nonnative neighborhoods. Mean seed set of common woolly sunflower (40–55 seeds/capitulum) was highest in nonnative neighborhoods, which was significantly greater than seed set in clipped nonnative neighborhoods (P < 0.001) and greater, but not significantly so, in native neighborhoods (P = 0.28). Researchers concluded that in nonnative neighborhoods hairy cat’s ear reduced the seed set of cutleaf silverpuff while facilitating pollinator visitation and seed set for common woolly sunflower (Waters et al. 2014).

Figure 9. Close up of a single common woolly sunflower flower from a plant growing in California. Photo: BLM CA180 SOS.
Ecology
Common woolly sunflower occurs in early-seral communities (Titus et al. 1998) and late-seral communities as long as there are canopy openings. It is common in disturbed habitats (Mooring 1975, 2001; Pavek 2011) and can persist for decades (Mooring 1975, 2001).
Most common woolly sunflower populations consist of 10 to 50 mature plants, although population size can vary four–fold depending on the year and is often greater in disturbed areas (Mooring 1975, 2001). Seed production is often prolific, which allows for rapid establishment in open sites (Pavek 2011). Common woolly sunflower occurred on several sites within the blast zone following the 1980 eruption of Mount St. Helens in Washington. The blast zone was revisited in 1993 and 1994. Common woolly sunflower was most common in refugia areas where some vegetation survived the blast, but it also occurred, albeit infrequently, on primary succession habitats formed by avalanches, debris deposits, landslides, and sites covered by pumice 30 to 660 ft (10–200 m) deep (Titus et al. 1998).
Seed And Seedling Ecology
Wildlife (insects, birds, and mammals) may be important to common woolly sunflower seed production and dispersal. According to Mooring (2001), insects damage more plant foliage than mammals, and fly and beetle larvae can destroy up to 80% of fruits. When Diaz (2020) investigated patterns of seed dispersal where both achillioides and leucophyllum varieties occurred in southern Oregon, biotic vectors were considered important to dispersal. Seed movement by biotic vectors was important to the persistence of polyploidy populations. Seed dispersal was best explained by landscape features (canopy and elevation) and not geographic distance (Diaz 2020).
In eastern Oregon, common woolly sunflower seed banks were only found where common woolly sunflower plants occurred in the aboveground vegetation (Carr and Krueger 2012). Seed bank samples were collected from 14 ponderosa pine stands, seven with an intact understory and seven with a depauperate understory. Common woolly sunflower was restricted to stands with an intact understory (14% frequency), where its density in the seed bank was 0.8 seed/ft² (9 seeds/m²). Soil samples (1.5 in³ [25.3 cm³] at up to 2 in [5 cm] depths) were collected from each stand and seed bank composition was determined using the emergence method in growth chambers (14 hrs light at 70 °F [20 °C], 10 hrs dark at 40 °F [5 °C]) (Carr and Krueger 2012).
Studies suggest that common woolly sunflower seedlings grow quickly under ideal conditions. In the greenhouse, the relative growth rate (RGR) of seedlings averaged 0.06 g/g/d at 14 days, 0.12 g/g/d at 28 days, and 0.10 g/g/d at 42 days after the appearance of cotyledons. Common woolly sunflower’s RGR was lower, although not significantly, than those of the invasive species investigated, which ranged from 0.11–0.15 g/g/d at 42 days (spotted knapweed [Centaurea stoebe], rush skeletonweed [Chondrilla juncea], Dalmatian toadflax [Linaria dalmatica], whitetop [Cardaria draba], Fuller’s teasel [Dipsacus fullonum], and Scotch cottonthistle [Onopordum acanthium]) (James and Drenovsky 2007).
Disturbance Ecology
Common woolly sunflower prefers full sun habitats and often increases with overstory removal. It was associated with dry, unshaded conditions when thinned, burned, and thinned and burned plots were compared in dry, low-elevation, ponderosa pine-Douglas-fir (Pseudotsuga menziesii) forests in northeastern Oregon (Youngblood et al. 2006). In the Skokomish River Basin, Washington, common woolly sunflower was an indicator of prairie vegetation when the composition of prairies, savannas, and woodlands was compared (Peter and Shebitz 2006). When ecological conditions were compared across the natural distribution of redwood forests, common woolly sunflower was most common at sites with the second highest light level (mean 34% full light) (Waring and Major 1964). In wetland prairies in Oregon’s Willamette Valley, common woolly sunflower increased when all woody vegetation (Saskatoon serviceberry [Amelanchier alnifolia], hawthorn [Crataegus spp.]), and blackberry [Rubus spp.]) was cut to ground level. Woody vegetation was removed in early October 1994 and again in late September 1996. Average cover increase for common woolly sunflower was 0.12% in 1995 and 0.47% in 1997 on treated plots. Average cover decrease was 0.16% in 1995 and 0.10% in 1997 on untreated plots (Clark and Wilson 2001).
Several studies suggest common woolly sunflower tolerates disturbance. When dry subalpine meadows with and without Olympic marmot (Marmota olympus) activity (i.e., mounding activity) were compared in Olympic National Park, Washington, the relative cover of common woolly sunflower was 1.3% on plots lacking mounds and 3.6% on plots with mounds (del Moral 1984).
Cover changes were higher on burned and woody vegetation removal plots than on untreated or mowed plots in a remnant wetland prairie in west Eugene, Oregon. Prescribed fire, hand removal of woody vegetation, and mowing and removal of cut material treatments were repeatedly applied to plots in late summer or early fall in 1994, 1996, 1998, and 2000 (Table 3; Clark 2002).
Table 3. Proportional change in cover of common woolly sunflower by treatment and year in remnant prairie vegetation near Eugene, OR (Clark 2002).
|
Treatment* |
Burned |
Hand removal |
Mowed |
Control |
|
Change in cover (%) |
||||
|
1995 |
0 |
+0.12 |
0 |
-0.16 |
|
1997 |
0 |
+0.47 |
0 |
-0.16 |
|
1999 |
+0.47 |
+0.55 |
0 |
+0.35 |
|
2001 |
+1.67 |
+0.83 |
0 |
+0.55 |
*Cover of common woolly sunflower before treatments was: 0 for burned, 0.9% for hand removal, 0 for mowed, and 0.5% for control.
Common woolly sunflower was nearly continuously distributed throughout Idaho fescue-dominated prairies at Fort Lewis, Washington, managed under a 3- to 5-year spring or fall burn cycle (Tveten and Fonda 1999). Common woolly sunflower was one of several indicator species on 22-year-old burned mixed-conifer forests in California’s Eldorado National Forest (Bohlman et al. 2016). The wildfire occurred in 1992 and post-fire sampling was concentrated in severely burned areas with more than 75% canopy tree mortality. Post-fire sites were also treated to reduce shrub cover and had trees planted following the fire. Common woolly sunflower did not occur on burned plots that regenerated without intervention (Bohlman et al. 2016). Density of common woolly sunflower increases from prefire levels were more substantial 1 to 3 years after fire (density increases of up to 5.6 times) than 4 to 5 years following fire (density increases of about 2 times) in chamise (Adenostoma fasciculatum) vegetation in Mendocino County, California (Sampson 1944).
Although most studies reported greater common woolly sunflower abundance on burned plots, that was not the case in the southern Willamette Valley. Common woolly sunflower frequency increased significantly on unburned plots (P < 0.1) but was unchanged on native wetland prairie plots that were fall burned once or twice and evaluated one year following fire (Pendergrass 1996).
Wildlife And Livestock Use
Common woolly sunflower is eaten by small mammals and is important to greater sage-grouse (Centrocercus urophasianus) and many insects and pollinators. In the Rattlesnake Hills in Richland, Washington, common woolly sunflower was recovered from Townsend’s ground squirrel’s (Urocitellus townsendii) fecal pellets (<1% relative frequency) (Rogers and Gano 1980). The Vancouver Island marmot (Marmota vancouverensis) feeds on common woolly sunflower leaves and flowers (Heard 1977, Milko 1984, cited in Nagorsen [1987]). In field studies, researchers found that gray-tailed voles (Microtus canicaudus) were specifically attracted to common woolly sunflower. Researchers compared the survival of greenhouse-grown, hemiparasitic, golden paintbrush (Castilleja levisecta) plants when planted with common woolly sunflower or Roemer’s fescue (Festuca idahoensis subsp. roemeri) host plants. Vole tunneling and feeding reduced survival of golden Indian paintbrush when it was planted with common woolly sunflower alone (66.7%) or when planted with both hosts (54.4%). Where golden Indian paintbrush was planted with Roemer’s fescue or without any hosts, survival rates were much higher and there was less rodent tunneling. Researchers also noted that voles were not targeting potting soil but were attracted to common woolly sunflower roots (Lawrence and Kaye 2008).
Common woolly sunflower supports a diversity of invertebrates important to greater sage-grouse. Greater sage-grouse likely also feeds on common woolly sunflower blooms in summer and in the late brood-rearing season (Luna et al. 2018). Various moths, including Agonopterix sabulella, Amblyptillia pica, Pelochrista maculatana, Phtheochroa aegrana, Phymatopus californicus, Sparganothis tunicana, and Telethusia ovalis use common woolly sunflower as a caterpillar host plant (NWF 2021). It is also a host plant for plant bugs (Coquillettia attica and Plagiognathus verticalis) (Schuh 2001; Wyniger 2011) and aphids (Macrosiphoniella sunshine) (Jensen et al. 2020). Aphids emerge in spring from overwintered leaves and move to the developing flower stems of perennials plants growing in Washington, Oregon, California, and perhaps beyond (Jensen et al. 2020).
Common woolly sunflower is a nectar source for orange sulfur (Colias eurytheme), Fender’s blue (Icaricia icarioides fender), Puget blue (I. i. blackmorei) red admiral (Vanessa atalanta), comma (Polygonia c-album), and skipper (Hesperiidae) butterflies (Schultz and Katrina 1999; LaBar and Schultz 2012; LBJWC 2016).
Nutritional Value
The nectar content of common woolly sunflower was evaluated for plants growing in four upland prairie sites in western Oregon where it was utilized as a nectar source for Fender’s blue butterfly. Of the 18 forbs analyzed, common woolly sunflower ranked eleventh best in sugar/flower nectar quantities and seventh best in sugar/flower unit nectar quantities (Table 4; Schultz and Katrina 1999).
Table 4. Average nectar quantities for common woolly sunflowers growing at four upland prairie sites in western OR (Schultz and Katrina 1999).
|
Flowers analyzed (no.) |
Sugar (mg)/flower |
Days individual flowers open |
Flowers/head |
Sugar (mg)/flower head |
|
37 |
0.057 |
1 |
67.8 |
3.87 |
Ethnobotany
The Native American Ethnobotany Database (Moerman 2003) reports that common woolly sunflower was used medicinally and ceremonially. The Chehalis used dried flowers as a love charm. The Skagit rubbed leaves on their faces to prevent chapping (Gunther 1973, cited in Moerman [2003]). The Miwok applied a poultice of leaves to soothe body aches and pains (Barrett and Gifford 1933, cited in Moerman [2003]).
Current Medicinal Use
High concentrations of thiarubrines and thiophenes, which have antimicrobial, anti-inflammatory, antihypertensive, and antitumor properties, occurred in common woolly sunflower roots from Hornby Island, British Columbia (Page et al. 1997).
Horticulture
Common woolly sunflower is a commercially available garden plant (Parkinson 2003; WY Extension 2021). It is an attractive plant that transplants well and grows in poor, moist to droughty soils (Parkinson 2003; Dorn and Dorn 2007; WY Extension 2021). It attracts a variety of pollinators and butterflies and is often recommended for borders and rock gardens (Dorn and Dorn 2007; Pavek 2011; LBJWC 2016). Planting in groups and pruning dead branches and dried flowers is suggested for the best appearance (LBJWC 2016; WY Extension 2021).
Common woolly sunflower was one of several species evaluated for use in green roof plantings in a study conducted at Oregon State University in Corvallis, Oregon. Researchers noted that common woolly sunflower in roof plantings was much less drought tolerant than it is in native habitats. Survivorship was 88% when 0.12 in (0.3 cm) of water was applied every 2 days (5.25 in [13.3 cm] total), 31% when 0.12 in (0.3 cm) was applied every 5 days (2.25 in [5.7 cm] total), and 6% without irrigation (1.5 in [3.8 cm] natural ppt.). Common woolly sunflower plants were planted on July 2007. The three irrigation treatments were initiated June 2008, and survival was evaluated 3 months later (Schroll et al. 2011).
Revegetation Use
There are several reports of the successful use of common woolly sunflower in revegetation (see Wildland Seeding and Planting section). It establishes and grows quickly to provide erosion control (Archibald 2006) and pollinator habitat (Ogle et al. 2011). Landis et al. (2005) referred to it as a “workhorse” species in roadside revegetation. Common woolly sunflower has high drought tolerance and grows in fine, medium, or coarse soils from sea level to 10,000 ft (3,050 m) in elevation where annual precipitation averages 9 in (23 cm) or more (Ogle et al. 2011; Pavek 2011).
Common woolly sunflower was successfully seeded as part of a pollinator mix in a vineyard in Polk County, Oregon. It worked well in this setting because its low stature did not interfere with airflow through the vines. It provides flowers and plant cover for multiple years and does not add nitrogen to the soil, which would adversely affect the grape quality (Bartow 2018b).
Developing A Seed Supply
For restoration to be successful, the right seed needs to be planted in the right place at the right time. Coordinated planning and cooperation is required among partners to first select appropriate species and seed sources and then properly collect, grow, certify, clean, store, and distribute seed for restoration (PCA 2015).
Developing a seed supply begins with seed collection from native stands. Collection sites are determined by current or projected revegetation requirements and goals. Production of nursery stock requires less seed than large-scale seeding operations, which may require establishment of agricultural seed production fields. Regardless of the size and complexity of any revegetation effort, seed certification is essential for tracking seed origin from collection through use (UCIA 2015).
Seed Sourcing
Common garden studies suggest the existence of large–scale geographic differences in common woolly sunflower genetics (Ward et al. 2008; Miller et al. 2011). When spatially- and temporally (3 yrs)-diverse seed collections were grown together at the Natural Resources Conservation Service’s Corvallis Plant Material Center (PMC), plants from seed collected from northern populations had silvery lanate pubescence, flowered earlier, and produced lighter colored flowers and seed pods than plants from seed collected from southern populations. Common woolly sunflower seed was collected from throughout the Willamette Valley and over a 3-year period (Ward et al. 2008). In other common garden studies conducted at Corvallis PMC, seed collected and grown from 19 populations from the Olympic Mountains, Washington, to the Umpqua Valley, Oregon, revealed that similarities between populations decreased with increasing geographic distance. Yet, researchers noted that this finding may have related to ploidy levels and soil type. Seed was sown in cone-tainers, and seedlings emerged from 56% of the cone-tainers (319 individual plants). These plants were evaluated in the common garden for size, emergence date, and flowering characteristics (Miller et al. 2011).
Because empirical seed zones are not currently available for common woolly sunflower, generalized provisional seed zones developed by Bower et al. (2014) may be used to select and deploy seed sources. These provisional seed zones identify areas of climatic similarity with comparable winter minimum temperature and aridity (annual heat:moisture index). In Figure 10, Omernik Level III Ecoregions (Omernik 1987) overlay the provisional seed zones to identify climatically similar but ecologically different areas. For site–specific disturbance regimes and restoration objectives, seed collection locations within a seed zone and ecoregion may be further limited by elevation, soil type, or other factors.
The Western Wildland Environmental Threat Assessment Center’s (USFS WWETAC 2017) Threat and Resource Mapping (TRM) Seed Zone application provides links to interactive mapping features useful for seed collection and deployment planning. The Climate Smart Restoration Tool (Richardson et al. 2020) can also guide revegetation planning, seed collection, and seed deployment, particularly when addressing climate change considerations.
Occurrence Map
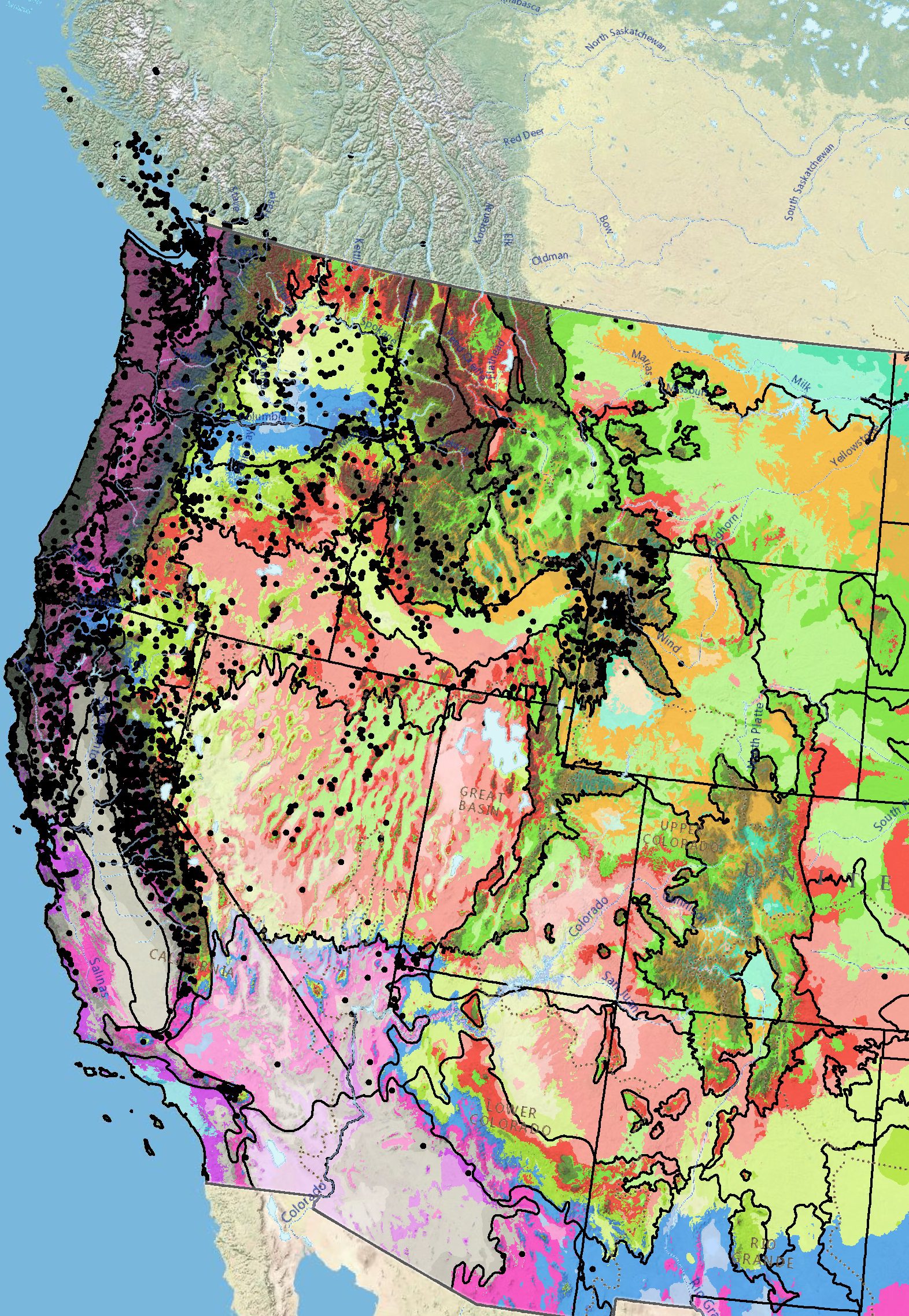
Figure 10. Distribution of common woolly sunflower (black circles) based on geo-referenced herbarium specimens and observational data from 1854–2018 (CPNWH 2020; SEINet 2020; USDI USGS 2020). Generalized provisional seed zones (colored regions) (Bower et al. 2014) are overlain by Omernik Level III Ecoregions (black outlines) (Omernik 1987; USDI EPA 2018). Interactive maps, legends, and a mobile app are available (USFS WWETAC 2017; www.fs.fed.us/wwetac/threat-map/TRMSeedZoneMapper2.php?). Map prepared by M. Fisk, USDI USGS.
Releases
As of 2021, there have been no common woolly sunflower germplasm releases.
Wildland Seed Collection
Common woolly sunflower plants are easy to identify. Seed is retained in the inflorescence longer than for many other Asteraceae, and seed is not wind-dispersed (Fig. 11; Skinner 2007; Bartow 2015). Low seed fill often requires large collections to meet seed needs (Bartow 2015).

Figure 11. Common woolly sunflower seed head, opened to show individual seeds. Photo: BLM OR110 SOS.
Wildland Seed Certification
Verification of species and tracking of geographic source is necessary whether wildland seed is collected for immediate project use or as stock seed for cultivated increase. This official Source Identification process can be accomplished by following procedures established by the Association of Official Seed Certifying Agencies (AOSCA) Pre-Variety Germplasm Program (Young et al. 2020; UCIA 2015). Wildland seed collectors should become acquainted with state certification agency procedures, regulations, and deadlines in the states where they collect.
If wildland-collected seed is to be sold for direct use in ecological restoration projects, collectors must apply for Source-Identified certification prior to making collections. Pre-collection applications, site inspections, and species and seed amount verification are handled by the AOSCA member state agency where seed collections will be made (see listings at AOSCA.org).
If wildland seed collected by a grower or private collector is to be used as stock seed for planting cultivated seed fields or for nursery propagation (See Agricultural Seed Field Certification section), detailed information regarding collection site and collecting procedures must be provided when applying for certification. Photos and herbarium specimens may be required. Germplasm accessions acquired within established protocols of recognized public agencies, however, are normally eligible to enter the certification process as stock seed without routine certification agency site inspections. For contract grow-outs, however, this collection site information must be provided to the grower to enable certification.
Collection Timing
Seed is often mature in July or August, but timing depends on elevation (Pavek 2011). Mature seeds are stiff and dark grayish brown to nearly black. Seed shatters within about a week of ripening (Mooring 2001; Skinner 2007).
The Bureau of Land Management’s Seeds of Success collection crews made 61 common woolly sunflower harvests from 2000 to 2020 in Oregon (63%), Washington (4%), California (5%), Idaho (19%), and Nevada (9%). Most collections were made in July (53%) and August (38%). The earliest collection was made on June 3, 2013, in El Dorado County, California, at 2,000 ft (610 m) elevation. The latest collection was made on September 8, 2009, from Jackson County, Oregon, at 4,936 ft (1,504 m) elevation. In the single year (2010) with the greatest number of collections (23), the earliest collection was made on July 3 in Malheur County, Oregon, at 3,747 ft (1,142 m) elevation. The latest was on August 31 in Klamath County, Oregon, at 4,876 ft (1,486 m) elevation (USDI BLM SOS 2017).
Collection Methods
Practitioners suggest clipping entire seed heads into containers (Fig. 12; Leigh et al. 2006; Skinner 2007).
Several collection guidelines and methods should be followed to maximize the genetic diversity of wildland collections: 1) collect seed from a minimum of 50 randomly selected plants; 2) collect from widely separated individuals throughout a population without favoring the most robust or avoiding small stature plants; and 3) collect from all microsites including habitat edges (Basey et al. 2015). General collecting recommendations and guidelines are provided in online manuals (e.g., ENSCONET 2009; USDI BLM SOS 2021).
It is critical that wildland seed collection does not impact the sustainability of native plant populations. Collectors should take no more than 20% of the viable seed available at the time of harvest (USDI BLM SOS 2021). Additionally, care must be taken to avoid the inadvertent collection of weedy species, particularly those that produce seeds similar in shape and size to those of common woolly sunflower.

Figure 12. Collection of common woolly sunflower seed heads harvested by clipping stems. Photo: BLM CA320 SOS.
Post-Collection Management
Skinner (2007) stored seed collections in paper bags at room temperature until cleaning. Large collections may require time on drying racks. Collections should be protected from rodents and inspected for insects.
Seed Processing/Storage. Large seed lot collections made at JHS Nursery were dried in bins (4 × 4 × 1.5 ft [1.2 × 1.2 × 0.5 m]) with fine mesh screens to allow air circulation. Bins were stacked six high with warm (100 °F [38 °C]) air blowing up through the bins. After 12 hours, seed moisture content was measured, and once it measured 5 to 8%, seed was packaged in plastic bags, which were put in boxes and stored cold (33–35 °F [0–2 °C]). Common woolly sunflower seed retains viability for many years (Archibald 2006, see also Seed Storage section).
Seed Cleaning
Processes used to clean small and large seed lots are presented below. Filled and unfilled seed can be difficult to distinguish, and there can be a high percentage of unfilled seeds (Wall and MacDonald 2009; Bartow 2015). Respiratory equipment is recommended when working with seed lots containing a lot of plant material, which is a lung irritant when crushed (Bartow 2015).
Small seed lots can be cleaned by first rubbing flower heads or blending whole flower heads in a standard house blender to separate the seed from the seed heads. Dry seed lots are then sifted or processed through an air column separator to separate filled seed from chaff and unfilled seed (Leigh et al. 2006; Skinner 2007). An indent cylinder is useful for removal of small weed seeds and other debris (Bartow 2015).
At the Rancho Santa Ana Botanic Garden, common woolly sunflower seed lots were gently rubbed on a rubber mat to break up chaff and flowers, then sifted through #12 and #30 sieves with a 1.1 to 1.25 blower speed. These processes removed about 75% of sterile seeds. Seed lots took 3 to 5 hours to process, and those with a high percentage of unfilled seeds required hand sorting (Wall and MacDonald 2009).
The USDA Forest Service, Bend Seed Extractory (BSE), cleaned a small seed lot (10.9 lbs [4.9 kg]) by first hand rubbing to separate seeds from the flower heads. The lot was then hand-screened using a 1/12 round screen to remove stems and chaff. Next, the lot was air screened to remove the remaining nonviable seed and inert material using an air-screen separator with a 1/22 × 1/2 slot top screen, a 1/25 round screen, medium speed, and low to medium air. Air screening was done a second time using a 1/25 × 1/2 slot top screen (Barner 2009a).
The BSE cleaned a large lot (330 lbs [150 kg]) by first air screening twice to remove stems and chaff. This was done using a Clipper Eclipse Model 324 (Hoffman Manufacturing Inc., Corvallis, OR) with a 5 round top screen, blank bottom screen, and medium to high air. The lot was finished using an Oliver Model 30 gravity separator (Oliver Manufacturing Co., La Junta, CO) at the following settings: speed of 70, air at 38, hopper speed of 1.5. This final process removes the remaining nonviable seed and other inert material. Processing of this seed lot skipped the usual first step of processing using a Westrup model HA 400 brush machine (Westrup, Slagelse, Denmark), because the seed had already been removed from the flower head (Barner 2009b).
Seed Storage
Common woolly sunflower seed is orthodox (RBG Kew 2021). Some seed stored at room temperature will germinate after as many as 8 years, but germination percentages decrease sharply after 2 years (Mooring 1975, 2001). The Pullman PMC stored clean seed at 40 °F (4 °C) and 40% relative humidity (Skinner 2007).
Seed Testing
There were no rules or guidelines for testing common woolly sunflower viability or germination in the available literature.
Germination Biology
Common woolly sunflower requires stratification for germination. At the Pullman PMC, common woolly sunflower did not germinate without cold, moist stratification (Skinner 2007). Germination averaged 10% after 45 days of stratification and 75% after 90 days of stratification. Some seed germinated during stratification, suggesting some emergence will occur at low temperatures. Containers sown in November and left outside through winter showed 82% germination during cool, fluctuating spring temperatures. Afterripening was not a replacement for stratification. Seed stored at (41 °F [5 °C]) and 40% relative humidity for 1 year failed to emerge without cold, moist stratification (Skinner 2007).
Common woolly sunflower seed harvested from commercial seed production plots in Willamette Valley, Oregon, germinated best after long-duration cold stratification (Russell 2011). Germination after 190 days of cold, moist stratification (66%) was almost double that with 61 or 98 days of stratification (38–48%) (P = 0.02). Seed was afterripened for at least 3 months at room temperature before germination experiments. The stratification temperature was (41 °F [5 °C]). Incubation occurred in a greenhouse at 59 to 77 °F (15–25 °C). There were germination differences between the two seed lots evaluated (Russell 2011).
Studies indicate that common woolly sunflower germinates at cool temperatures, although germination takes longer at cold than warm incubation temperatures. For seed collected near Tacoma, Washington, germination without any pretreatment occurred after 9 days at warm temperatures and after 17 days at cold temperatures (Drake and Ewing no date). For seed that was cold stratified at 36 to 43 °F (2–6 °C) for 6 weeks, germination occurred after 6 days when incubated at warm temperatures and after 15 days when incubated at cold temperatures. First germination took slightly longer for seeds that were cold stratified for 12 weeks, which was 12 days with warm incubation and 19 days with cold incubation. Seed was cold stratified in a moist, sterile, inorganic soil mix. Warm incubation occurred in a greenhouse with 65 to 70 °F (18–21 °C) nighttime temperatures and 70 to 85 °F (21–29 °C) daytime temperatures. Cold incubation occurred outdoors where temperatures ranged from 48 to 65 °F (8–18 °C). In this study, longer duration stratification did not improve germination, and maximum germination was 31% after 6 weeks of stratification regardless of incubation temperatures (Drake and Ewing no date). When incubation temperatures of 34 to 100 °F (1–38 °C) were tested for untreated seeds, 69 °F (20.5 °C) was calculated as the optimal germination temperature for common woolly sunflower (Russell 2010).
Wildland Seed Yield And Quality
Post-cleaning seed yield and quality of seed lots collected in the Intermountain region are provided in Table 5 (USFS BSE 2017). The results indicate that common woolly sunflower seed can be cleaned to high levels of purity and seed fill but that the purity and fill of fresh seed collections can be highly variable. Seed weight varies by variety. RGB Kew (2020) reported 1,757,809 seeds/lb (3,875,240 seeds/kg) for variety arachnoideum, 1,054,685 seeds/lb (2,325,143 seeds/kg) for lanceolatum, and 909,941 seeds/lb (2,006,043 seeds/kg) for variety integrifolium (RBG Kew 2020).
Table 5. Seed yield and quality of common woolly sunflower seed lots collected in the Intermountain region, cleaned by the Bend Seed Extractory, and tested by the Oregon State Seed Laboratory or the USFS National Seed Laboratory (USFS BSE 2017).
| Seed lot characteristic | Mean | Range | Samples (no.) |
| Bulk weight (lbs) | 2.5 | 0.01–64.8 | 124 |
| Clean weight (lbs) | 0.5 | 0.006–9.0 | 124 |
| Clean-out ratio | 0.2 | 0.016–0.37 | 124 |
| Purity (%) | 95 | 29–99 | 123 |
| Fill (%)¹ | 93 | 32–99 | 124 |
| Viability (%)² | 91 | 73–98 | 70 |
| Seeds/lb | 1,170,630 | 111,669–14,000,000 | 123 |
| Pure live seeds/lb | 991,904 | 680,300–1,395,836 | 70 |
¹100 seed X-ray test
²Tetrazolium chloride test
Marketing Standards
Acceptable seed purity, viability, and germination specifications vary with revegetation plans. Purity needs are highest for precision seeding equipment used in nurseries, while some rangeland seeding equipment handles less clean seed quite well.
Agricultural Seed Production
Common woolly sunflower has been grown agriculturally for seed production by several organizations. The Corvallis PMC reports easy establishment from seed and many years of seed production from agricultural fields (Bartow 2015). Although plants often flower the first year, first-year seed production is limited (Pavek 2011; Bartow 2015). Heritage Seedlings Inc. reports high seed yields (400 lbs/ac [450 kg/ha]), relatively high seed viability (90%), and that seeding, harvesting, and cleaning can be done mechanically (Boyer 2008).
Agricultural Seed Certification
In order to minimize genetic changes in specific accessions of native species when increased in cultivated fields, it is essential to track the geographic source and prevent inadvertent hybridization or selection pressure. This is accomplished by following third party seed certification protocols for Pre-Variety Germplasm (PVG) as established by the Association of Official Seed Certification Agencies (AOSCA). AOSCA members in the U.S., Canada, and other countries administer PVG requirements and standards that track the source and generation of planting stock. Field and cleaning facility inspections then monitor stand establishment, proper isolation distances, control of prohibited weeds, seed harvesting, cleaning, sampling, testing, and labeling for commercial sales (Young et al. 2020; UCIA 2015).
Seed growers apply for certification of their production fields prior to planting and plant only certified stock seed of an allowed generation (usually less than four). The systematic and sequential tracking through the certification process requires preplanning, knowing state regulations and deadlines, and is most smoothly navigated by working closely with state certification agency personnel. See the Wildland Seed Certification section for more information on stock seed sourcing.
Site Preparation
Common woolly sunflower was grown for seed production at the USDA Forest Service’s J. Herbert Stone (JHS) Nursery in southwestern Oregon (Archibald 2006). At JHS Nursery, the growing season is long, climate is dry, and soils are deep, well–drained with high fertility and a pH of 5.5 to 6.0. Seedbed preparation at JHS Nursery included soil fumigation in late August or early September using dazomet (350 lb/ac [392 kg/ha]). Fields were ripped, disked, and then formed into 4-ft (1.2-m) wide raised beds. Beds were broadcast fertilized with a mix of ammonium phosphate and potassium sulfate (250 lbs/ac [280 kg/ha]/each) prior to seeding (Archibald 2006).
Seed Pretreatments
In seeding experiments conducted at Oregon State University’s Malheur Experiment Station (OSU MES), pretreating seeds with a mixture of fungicides to prevent seed decomposition and seedling damping off improved stand emergence (Shock et al. 2014). For more information on this study, see the Seeding section below.
Weed Management
A variety of weed management tools and methods were used to control weeds in common woolly sunflower seed production fields (Archibald 2006; Bartow 2015). Hand hoeing, spot herbicide treatments, row tillage, and mowing were used at the Corvallis PMC. When annual weed competition was high in the establishment year, entire fields were fall mowed using a flail mower or shredder and then vacuumed to remove annual weed seed. Common woolly sunflower plants grew back quickly following mowing (Bartow 2015). JHS Nursery indicated that weed control was the biggest challenge for multiple years of seed production (≤ 5 yrs). Soil was fumigated before seeding to promote dense stands and limit weed germination. Weed seed inputs were reduced by mowing, cultivating, or chemically treating weeds surrounding the nursery fields, in tractor paths, and between rows. Hand removal was the primary control of weeds within beds. Hand removal of weeds was effective but costly (Archibald 2006).
Several studies reported on the susceptibility and resistance of common woolly sunflower to pre- and post-emergent herbicides. In field evaluations of preemergent herbicides at OSU MES, none of the preemergent herbicides significantly reduced common woolly sunflower emergence when compared to untreated emergence. Preemergent herbicides tested were: pendimethalin, dimethenamid-P, S-ethyl dipropylthiocarbamate, S-metolachlor, benefin, and bensulide (Shock et al. 2014). Effects of post-emergent herbicides were also field tested at OSU MES. Octanoic acid ester of bromoxynil and carfentrazone-ethyl herbicides caused significant injury and stand loss when compared to untreated controls. Oxyfluorfen, clethodim, pendimethalin, and dimethenamid-P herbicides did not result in greater injury or stand loss than untreated control plots (Shock et al. 2014). When greenhouse-grown seedlings were evaluated for herbicide resistance, common woolly sunflower was resistant to the broadleaf-specific tribenuron, showing no dry weight reduction after exposure to the field application rate. It was also resistant to the grass-specific fluazifop herbicide. Glyphosate treatments at the recommended field application rate significantly reduced the dry weight of common woolly sunflower (P < 0.1) (Blakeley-Smith 2006).
Seeding
Because most common woolly sunflower populations have high percentages of dormant seed, it should be sown in the fall (Bartow 2015). Seed should be shallowly buried (<0.33 in [0.8 cm]) and seeded at a rate of 3 to 4 lbs/ac (3.4–4.5 kg/ha) (Archibald 2006; Bartow 2015). At JHS Nursery, seedbeds were fall sown at a rate to achieve 12 plants/ft² (130 plants/m²). Seed was placed in bands that were 0.75 in (1.9 cm) deep, 1.25 in (3 cm) wide, and 12 in (30 cm) apart using a Love/Oyjord drill (Garfield, WA) with packing wheels that pressed seed into the soil. Seed was covered with 0.25 to 0.33 in (0.6–0.8 cm) of sawdust and kept moist until fall rains began. Different seedlot locations were kept 150 ft (45 m) apart (Archibald 2006).
At OSU MES, researchers evaluated seven planting systems with common woolly sunflower (Shock et al. 2014). Emergence was evaluated early in the first spring following fall seeding. Row cover was most important to improving the percent of stand emergence. Without row cover, stand emergence was less than 15%. Treating seeds with a mixture of fungicides to prevent seed decomposition and seedling damping off also improved stand emergence. With row cover and seed pretreatment, stand emergence was 39 to 55%. With row cover but without seed pretreatment, stand emergence was 26 to 32% (Shock et al. 2014).
Establishment And Growth
When seeded in fall, common woolly sunflower readily germinates the following spring. Seedling vigor is considered high, and plants develop quickly (Bartow 2015). At Corvallis PMC, plots fertilized with nitrogen (50 lbs/ac [56 kg/ha]) in the spring were taller, produced more flowers, bloomed longer, and had higher amounts of filled seed than unfertilized plants. Plants tolerate low mowing in the fall without spring emergence problems if mowed residue is less than 4 in (10 cm) thick (Bartow 2015). At JHS Nursery, seedlings that germinated outdoors in the fall grew slowly to 2 to 3 in (5–8 cm) over winter. Seedling growth rates were rapid by early April. Plants flowered in May, at which point beds were fertilized twice with ammonium nitrate (100 lb/ac [112 kg/ha]) and irrigated frequently, which increased plant vigor and promoted seed production. Established plantings were fertilized in early spring with 250 to 300 lb/ac (303–336 kg/ha) of 13N:13 P2O5:13K2O. After seed harvest, fields are provided minimal irrigation until early fall when irrigation is applied to encourage root growth (Archibald 2006).
Pollinator Management
Common woolly sunflower is visited and pollinated by solitary, cavity-nesting mason bees (Osmia montanum and O. californica), which are readily managed (Cane 2011, personal communication in Pavek 2011). O. californica populations prospered from nesting blocks and as released populations at the Corvallis PMC, where they pollinated common woolly sunflower seed production fields (Cane et al. 2012).
Pest Management
Slugs can be serious pests for seedlings, and lygus bugs are a problem for flowering common woolly sunflower plants (Bartow 2015). Cercosporella cana, Puccinia eriophylli, and Ramularia eriophylli fungi were collected from wildland plants growing in Idaho, Oregon, or Montana (Farr and Rossman 2017). Yet, no powdery mildew was detected in seed production plots growing at OSU MES (Mohan and Shock 2014). No disease or insect problems were encountered in seed fields at JHS Nursery (Archibald 2006).
Seed Harvesting
The Corvallis PMC considers common woolly sunflower seed to be some of the easiest to harvest. Because seed does not shatter easily, it can be harvested mechanically (Bartow no date). Seed can be difficult to remove from seed heads without concurrent hot dry conditions (Bartow 2015).
Large fields were harvested by first swathing plants into a windrow, then combining after allowing plant material to dry as much as possible, which improved seed detachment from the seed heads. Smaller fields were harvested by machines that swath and collect plant material at the same time. Because plant material is a lung irritant when crushed, respiratory equipment is recommended during harvesting operations (Bartow 2015).
At JHS Nursery, common woolly sunflower seed lots are monitored weekly for seed ripeness then daily as seed lots mature (Archibald 2006). Harvest dates are set to maximize the collection of mature seed, which is done using a small-plot combine. A silage chopper follows combining to remove the remaining plant residue (Archibald 2006).
Seed Yields And Stand Life
Common woolly sunflower seed yields range from 100 to 400 lbs/ac (110–450 kg/ha) (Boyer 2008; Bartow 2015). Weak seed yields are common in the first year (Archibald 2006; Bartow 2015). At Corvallis PMC, production typically peaked in the fourth year, and stands were harvested for up to 7 years (Bartow 2015). At JHS Nursery, seed yield varied considerably depending on seed lot, growing season, and stand age. Stands were harvested for a maximum of 5 years (Archibald 2006).
Nursery Practice
Many researchers and practitioners grew common woolly sunflower plants under nursery conditions and shared their protocols. Mooring (1975) reported that plants live up to 6 years in the greenhouse.
For an herbicide-resistance experiment, common woolly sunflower was grown by sowing seed in seedling trays filled with a commercial seedling potting soil (OBC #1) (Blakeley-Smith 2006). Trays were cold stratified at 41 °F (5 °C) for 6 weeks then moved to a warm mist bench for 3 to 5 days. Seedlings grew there for 10 to 19 days before being transplanted to 2-in (10-cm) pots filled with OBC soil #3 with Osmocote fertilizer, where they grew for another 14 days (Blakeley-Smith 2006).
Common woolly sunflower seedlings grown for restoration of an oak savanna near Corvallis, Oregon, were grown by sowing seed into plug flats (1 × 2 in [2.5 × 5.1 cm]) filled with a 5:4:1 oak leaf mold, compost, and native topsoil mix (Vance et al. 2006). Flats were kept in an unheated greenhouse. Germination occurred in January and February. Irrigation was reduced starting in August to closer simulate savanna conditions. About 2 weeks before outplanting, plants were transplanted to slightly larger containers and irrigated daily to encourage root growth (Vance et al. 2006).
The Pullman PMC grew plants from seed collected south of Moscow, Idaho, to produce transplant plugs (Skinner 2007). Seed was sown directly into 10-in³ (164-cm³) cone-tainers filled with Sunshine Mix #4. Seed was covered lightly with soil and a layer of coarse grit to keep seeds in place during watering. Cone-tainers were watered deeply and placed outdoors until January when they were moved into the greenhouse. Soil was kept moist. Germination began within 3 days and was complete after 13 days in the greenhouse. Seedlings were watered deeply every other day and fertilized once each week with a water-soluble fertilizer containing micro-nutrients. In late March or early April, cone-tainers were moved to a cold frame where they were watered every other day in cool weather and every day in hot, dry weather. Plants were hardened for 2 to 4 weeks before outplanting (Skinner 2007).
Researchers at the University of California, Chico, suggested nursery production of common woolly sunflower may be improved by using native loam soils rather than potting soil, because native soils have lower moisture and temperature fluctuations (Leigh et al. 2006). At Chico, seed collected from east Tehama County, California, was sown in flats filled with a 1:1:1:2 sand, pumice, peat moss, and fir (Abies spp.) bark mixture. Flats were placed in a cold frame from late fall through spring. Transplantable sprouts were produced within 3 weeks of germination. Seedlings were transplanted into various sized pots using the same potting mix. Common woolly sunflower grew actively well into the summer. Excessive summer watering caused fungal growth and root rot (Leigh et al. 2006).
Wildland Seeding And Planting
Common woolly sunflower is a fast growing, persistent, pollinator plant useful for soil stabilization at sites with fine to coarse-textured soils receiving 9 to 25 in (229–635 mm) of annual precipitation (Ogle et al. 2011; Pavek et al. 2013). Drill seeding less than 0.25 to 0.5 in (0.6–1.3 cm) deep at rates of 3 to 4 lbs/ac (3.4–4.5 kg/ha) into a weed–free seed bed is recommended (Ogle et al. 2011; Pavek 2011; Pavek et al. 2013; Ogle et al. 2014).
Restoration Seedings. Many restoration experiments and projects have evaluated common woolly sunflower establishment and survival with varied seeding rates, seeding mixes, site preparation, and seeding methods. In general, higher seeding rates, site preparation that limited competition, and site preparation involving fire resulted in improved establishment and survival.
Common woolly sunflower establishment was limited in the western Willamette Valley, Oregon, when seeded at rate of 6 seeds/ft² (66 seeds/m²) on twice-tilled degraded wetland prairie sites (Wilson et al. 2004). Sites were tilled on May 12, 1992, using a three-point 48-in (122 cm) Howard HA Rotovator (Wiltshire, England) to kill existing weedy vegetation and stimulate germination of buried seed. Sites were tilled again to kill emerging seedlings. Common woolly sunflower was seeded as part of a native grass-forb mixture on October 21, 1992. Cover of common woolly sunflower was 0.2% cover in year 1 and 0.4% in year 2. Tests showed that common woolly sunflower seed viability was 26%, and germination was 18% (Wilson et al. 2004).
Common woolly sunflower cover was greatest on plots receiving the highest seeding rate in attempts to restore wildlife habitat in an abandoned pasture in Polk County, Oregon (Bartow 2018a). It was one of 15 forbs and 5 grasses seeded at total rates of 30, 50, and 70 seeds/ft² (320, 540, 750 seeds/m²). The site was glyphosate-treated fall of 2012, mowed the following spring, and glyphosate-treated three more times in 2013. The site was lightly disked in late summer 2013. Plots were seeded on October 22, 2013, using a precision cone-seeder, which seeded to a depth of less than 0.25 in (0.6 cm) in rows spaced 12 in (30 cm). Common woolly sunflower made up 8% of the seeding mix (by weight) and was seeded at rates of 2.4, 4 and 5.6 seeds/ft² (26, 43, 60 seeds/m²). Plots seeded at the highest rate had the most native forbs and fewest weeds. Forb cover was dominated by common woolly sunflower, common selfheal (Prunella vulgaris), western yarrow (Achillea millefolium), and checkermallow (Sidalcea campestris) (Bartow 2018a).
Establishment was not different when Cross Slot and Great Plains double disk no-till drills were used to seed mowed and unmowed sites in Latah County, Idaho (Pavek et al. 2016). A mixture of 17 forb species were seeded on October 21, 2010, on mowed and unmowed plots. Sites were glyphosate treated in June 2010, mowed in mid-October 2010, and clethodim treated again in spring 2011 and 2012. The seeding rate for common woolly sunflower was 3 PLS/linear ft (10 PLS/ linear m). Three years after seeding there were no consistent establishment differences among drill types or mowing. Density of common woolly sunflower was greatest at site two (~11 plants/linear ft [36/m]), lower at site three (~6 plants/linear ft [20/m]), and lowest at site one (<1 plant/linear ft [<3/m]). Site two was a 20-year-old stand of intermediate wheatgrass (Thinopyrum intermedium). Sites one and three were 7-year-old stands of bluebunch wheatgrass and Idaho fescue. Site one was seeded deeper (<0.25 in [0.6 cm]) than sites two and three. Researchers noted that weed control during and after seeding was essential to forb establishment success (Pavek et al. 2016).
Cover of common woolly sunflower was significantly greater when seeded a year before rather than at the same time as grasses or a year after grasses (P < 0.05) at Finley National Wildlife Refuge south of Corvallis, Oregon (Clark and Wilson 2005). The site was herbicide treated in November 2002 and tilled in October 2003. Seeding occurred in fall 2003 and 2004 at a density of 25 seeds/ft² (270 seeds/m²) for the grass and forb mixes. For plots seeded with grasses and forbs separately, plots were mowed to about 3 in (8 cm) and tilled in September 2004 prior to sowing rows at right angles to previous years’ seeding of grasses. Following these field studies, researchers suggested sowing forbs before grasses if the restoration goal is to maximize species richness and forb cover. In additional field experimentation, common woolly sunflower was fall sown as a monoculture (50 seeds/ft² [540/m²]) on plots prepared as described above. On these plots, cover of common woolly sunflower increased and nonnative species cover decreased over time. Cover of common woolly sunflower was 5% in 2004 and 16% in 2005; and nonnative cover was 42% in 2004 and 20% in 2005. Researchers suggested increasing the monoculture seeding rate to better minimize nonnative cover (Clark and Wilson 2005).
In prairie restoration conducted at the Glacial Heritage Preserve in Thurston County, Washington, common woolly sunflower leaf area when seeded in a 50:50 grass-forb ratio when three grass-forb seed counts (2:98, 25:75, 50:50) were compared (Mitchell and Bakker 2016). The study site was an old field dominated by nonnative grasses. It was glyphosate treated, tilled, and then covered with clear plastic for 3 months to solarize the soil. Seeding occurred in fall 2008 at rate of 1,970 seeds/linear ft (600 seeds/ linear m). Seeded species traits were evaluated in 2009. Height of common woolly sunflower plants did not differ consistently among the seeding ratios, but average specific leaf area was highest in the grass-rich (50:50) seed mix. Relative cover of common woolly sunflower in the restoration area was 4.3% (Mitchell and Bakker 2016).
When seeding rates and seeding methods were evaluated in the restoration of rare butterfly habitat in grassland sites in western Washington, common woolly sunflower density and cover increased with seeding rate (Applestein et al. 2018). Restoration sites were located within 25 miles (40 km) of each other, where annual precipitation averages 49 in (1,250 mm) and 75% occurs between October and March. Soils are deep, well-drained, gravelly sandy loams. Site preparation began with a grass-specific herbicide treatment in the spring, followed by a fall burn and a glyphosate treatment within 2 weeks of the fire. Sites were seeded in various combinations of forb and grass seed numbers in January or October of 2010. In January, plots were seeded with a 0.75:1 mix of Roemer’s fescue and common woolly sunflower seeds. In October, plots were seeded with a 3:1:1 mix of Roemer’s fescue, common woolly sunflower, and slender cinquefoil (Potentilla gracilis) seeds. Mixes were seeded using three methods: drill seeding using a Kasco no-till drill (Kasco Manufacturing Shelbyville, IN), broadcast seeding using a Trillion broadcast seeder (Truax Co, New Hope, MN), which was followed by raking with a harrow, and hydroseeding (Hydrostraw mulch added 2,000 lbs/ac [2,240 kg/ha]). Five total seeding rates (calculated in terms of bulk seed weight) were evaluated: 0, 32, 65, 98, and 130 seeds/ft² (350, 700, 1,050, and 1,400/m²). First year establishment of common woolly sunflower averaged 10%. Density of common woolly sunflower in year 1 was strongly related to seeding rate. Cover in year 3 was correlated with seeding rate. Researchers found no interaction between seeding rate and seeding method (Applestein et al. 2018).
Establishment of common woolly sunflower was slightly better on twice-burned than unburned sites in a nonnative grass-dominated Oregon white oak savanna in southeastern Vancouver Island, British Columbia (MacDougall and Turkington 2006). Each plot (6 ft² (0.6 m²) was seeded with a nine-species forb seed mix, thus 321 common woolly sunflower seeds were hand broadcasted on each plot. Seeding occurred on unburned plots in late July and on twice-burned plots in early October 2000. In 2005, cover of common woolly sunflower was 0.4% on unburned and 1.4% on burned plots (MacDougall and Turkington 2006).
Findings were similar when seeding was compared on burned and unburned plots (Maret and Wilson 2000). Common woolly sunflower was hand broadcast seeded alone at a rate of 100 seeds per 2 × 2–in (5 × 5–cm) subplots. Seedling emergence was greater on fall-burned than unburned plots and better in subplots dominated by native bunchgrasses (blue wildrye [Elymus glaucus], Roemer’s fescue) and annual nonnative grasses (medusahead [Taeniatherum caput-medusae]), than on subplots dominated by perennial nonnative grasses (orchardgrass [Dactylis glomerata]). When site preparation comparisons included burning, clipping, burning but adding litter, and no manipulation in the same study area, common woolly sunflower establishment was improved by burning in plots dominated by nonnative annual grasses (Table 6). Common woolly sunflower was one of several species evaluated separately by sowing 100 seeds/subplots (2 × 2 in [5 × 5 cm]) in late September (Maret and Wilson 2000).
Table 6. Average number of seedlings/subplot (2 × 2 in) the first spring following sowing in plots prepared by burning, clipping with litter removed, burning with litter added, and no manipulation (Maret and Wilson 2005).
|
Site |
Burned |
Clipped |
Burned, litter added |
Unmanipulated |
|
Annual nonnative grass |
17.0a |
7.8b |
8.0b |
3.1b |
|
Perennial nonnative grass |
10.9a |
5.0a |
4.8a |
6.0a |
|
Native bunchgrass |
22.9a |
9.7a |
12.5a |
9.9a |
Values within a row followed by different letters were significantly different (P < 0.05).
In field experiments to restore Fender’s blue butterfly habitat at a degraded upland prairie site near Eugene, Oregon, common woolly sunflower establishment and flower production were better at the more productive site prior to site preparation and seeding (Tables 7a, 7b; Schultz 2001). The Willow Creek site supported a high diversity of weeds and relatively high plant biomass. The Royal site had a low diversity of weeds, sparse grasses, and much lower biomass. The two sites were split into plots that were tilled, reverse fertilized, solarized, or unmanipulated. Reverse fertilization is the process of adding carbon to the soil to reduce available nitrogen. In this study, sugar was used as the carbon source, and it failed to reduce soil nitrogen. Solarization involved covering tilled plots with clear plastic through the hot summer months. Two seed mixes (by seed number) of four grass and seven forb species were tested: a 50:50 grass:forb mix and forbs and a 90:10 grass:forb mix based on relative number of seeds. Sites were watered (~10,000 gallons/each 30 × 92–ft [9 ×28–m] experimental block) before seeding with wild-collected seed in September 1995. The common woolly sunflower seeding rate was 3.3 seeds/ft² (36/m²) plot in the 50:50 mix and 42/ft² (450/m²) plot in the 90:10 mix. To reduce deer herbivory, the Willow Creek site was fenced in November 1995 and the Royal site in late April 1996. There were significantly more weeds at the Royal than the Willow site in all soil treatments (P < 0.0001). Common woolly sunflower established better and produced more flowers at the Willow Creek than Royal site (Tables 7a, 7b). At Willow Creek, establishment and survivorship of common woolly sunflower were significantly greater on tilled or solarized than control or reverse fertilized plots (P < 0.001), and establishment was significantly better from the high forb than low forb seed mix (P < 0.001) (Tables 7a, 7b; Schultz 2001).
Table 7a. Total number of common woolly sunflower plants/site (30 × 92 ft) following experimental restoration treatments at two upland prairie sites near Eugene, OR, that were seeded in September 1995 (Schultz 2001).
|
Site |
Total seed input (no.) |
1996 |
1997 |
1998 |
1999 |
|
Plants (no.) |
|||||
|
Willow Creek |
54,000 |
4495 |
1301 |
1122 |
1681 |
|
Royal |
54,000 |
932 |
38 |
73 |
75 |
Table 7b. Common woolly sunflower flower production/11 ft² 4 years following experimental restoration of upland prairie sites near Eugene, OR (Schultz 2001).
|
Site |
Control |
Reverse Fert |
Solarization |
Tilled |
|
Willow 50:50 grasses:forbs |
0 |
0.9 |
8.0 |
4.6 |
|
Royal 50:50 |
0 |
0.1 |
0.3 |
0 |
|
Willow 90:10 grasses:forbs |
0 |
2.0 |
2.3 |
2.7 |
|
Royal 90:10 |
0 |
0 |
0 |
0 |
Restoration plantings. In studies using common woolly sunflower plants in restoration, survival was often good, and plants typically produced seed soon after transplanting. Common woolly sunflower was one of several species planted following the removal of Douglas-fir and Scotch broom (Cytisus scoparius) and a prescribed fire at Rocky Prairie Natural Preserve, Washington. Idaho fescue and five forb species were planted 1 to 2 months following woody vegetation removal. Mortality of common woolly sunflower plants grown from locally-collected seed was 15% at 2 months after transplanting. In the following year, surviving plants were flowering and producing seed (Thomas and Gamon 1997).
Variety arachnoideum transplants were short-lived but reproduced readily and extended beyond originally planted sections in Mendocino County, California (Everett 1957). Transplants were evaluated for 7 years by the Rancho Santa Ana Botanic Garden. They were planted in April at a semi-shaded to shaded site on a north slope with dark, silty, clay soils. Plants grew up to 6 in (15 cm) tall and 12 in (30 cm) wide. Researchers harvested three lots of seed from the site (Everett 1957).
Common woolly sunflower transplant survival was high, and plants produced seed within 1 to 2 years of planting in a biscuit scabland native plant garden (Youtie 1992). Common woolly sunflower was planted along with other native grasses and forbs adjacent to the Governor Tom McCall Preserve between Hood River and The Dalles, Oregon. Seed was collected locally, refrigerated for several months, grown in a greenhouse, and acclimated in a cold frame. Outplanting occurred in early March, and transplants were watered once a week for first 6 weeks (Youtie 1992).
Common woolly sunflower survival was close to 50% three years following outplanting in an Oregon white oak savanna at Fort Hoskins Historic Park near Corvallis, Oregon (Vance et al. 2006). Survival and flowering were evaluated on unseeded plots and plots seeded with five native grasses. Unseeded plots were dominated by nonnative pasture grasses. Common woolly sunflower was planted in the fall of 2001, and transplants were protected from herbivory with plastic mesh tubes. Common woolly sunflower survival was about 80% in 2002, 60% in 2003, and 52% in 2004. There were very few flowers in 2002, but in 2004, flowering averaged 30%. Grass cover increased on seeded plots, which reduced forb cover but not survival. There was no significant difference in the survival of outplanted forbs between the seeded and unseeded plots (P > 0.05) (Vance et al. 2006).
Survival was close to 50% for common woolly sunflower 3 years after planting to restore an urban landfill (Table 8; Ewing 2002). The landfill in Seattle, Washington, was closed in 1966 and graded and seeded with nonnative pasture grasses in 1971. Seven native prairie plants were grown from locally-collected seed in 4-in (10-cm) pots. Outplanting occurred in May 1995 on plots with the following treatments each paired with a control: mounded (low sandy loam mounds about 8 in [20 cm] high and 7 ft [2 m] in diameter), fertilized (NPK 27-3-4 at 202 lbs/ac [226 kg/ha]), or composted (4 in [10 cm] of compost added) (Table 8; Ewing 2002).
Table 8. Survival of common woolly sunflower in the first 3 years on variously prepared plots on a reclaimed urban landfill in Seattle, Washington. Common woolly sunflower outplanted in May 1995 (Ewing 2002).
|
Year |
Base |
Amendment |
Mulch |
|||
|
Mounds |
None |
Fert |
None |
Compost |
None |
|
|
Survival (%) |
||||||
|
Sept 1995 |
98a |
97a |
99a |
96a |
97a |
98a |
|
July 1996 |
98a |
72b |
82a |
88a |
90a |
80a |
|
July 1997 |
64a |
32b |
46a |
51a |
51a |
46a |
Values within a single plot treatment and year followed by different letters are significantly different (P<0.01).
Restoration by targeting nonnative vegetation. Researchers reported that common woolly sunflower increased significantly on plots treated with an herbicide that targets grasses (Nyamai et al. 2011). The field trials were designed to restore prairie remnants in Latah County, Idaho. Prairie sites were treated with isopropylamine salt of imazapyr at a rate of 0.0491 gal/ac (0.459 L/ha) when cheatgrass (Bromus tectorum) and ventenata (Ventenata dubia) were at the one- to two-leaf stage). Herbicide treatments occurred alone and in conjunction with seeding of native species (three bunchgrasses, common yarrow [Achillea millefolium], and firecracker penstemon [Penstemon eatonii]), but abundance of common woolly sunflower was not reported for seeded and unseeded plots (Nyamai et al. 2011).
Appendices
Appendix 1. Characteristics of common woolly sunflower varieties (Munz and Keck 1973; Johnson and Mooring 2006; Hitchcock and Cronquist 2018).
|
Variety |
Life form* |
Root system |
Height (in) |
Heads |
Peduncles (in) |
Ray florets |
Cypselae (mm) |
Pappi (mm) |
Flowering date |
|
achillioides |
A–P |
± taproot |
6–28 |
usually 3–8/array; sometimes single |
1–4 |
usually 9–13, sometimes 0 |
2–3.3 |
0.3–1 |
April–July |
|
arachnoideum |
P, clumped |
taproot or roots branched, sometimes stoloniferous |
12–24 |
2–4 per array or borne singly |
1–4 |
8–15 |
1.8–4 |
0–0.2 |
April–Aug |
|
croceum |
P |
roots branched, often stoloniferous |
4–24 |
2–4/array or borne singly |
1–3 |
10–14 |
2–4 |
0–0.1 |
May–July |
|
grandiflorum |
P, sometimes flowers in yr 1 |
± taproot |
12–40 |
2–6/array, or single |
4–12 |
10–15 |
2.4–4 |
0.5–2 |
April–July |
|
hallii |
P |
± taproot |
2–4 per array or borne singly |
2–5 |
8–9 |
3.5–5 |
0.7–2 |
June–July |
|
|
integrifolium |
S, multistemmed |
taproot |
4–10 |
usually borne singly |
1–4 |
5–10, generally 8 |
2.3–5 |
0.3–2 |
June–Aug |
|
lanceolatum |
P |
± taproot |
8–22 |
usually borne singly, sometimes 2–3/array |
1–6 |
10–15 |
1.9–3.2 |
0.5–1.4 |
May–Aug |
|
lanatum |
P |
± taproot |
10–24 |
2–5/array or borne singly |
2–8 |
8 –13 |
3.4–5 |
0.4–1.8 |
May–June |
|
leucophyllum |
P, often clumped |
multi–branched fibrous roots |
8–24 |
2–5/array or borne singly |
1–5.5 |
8–13, sometimes 0 |
2–4 |
0.3–1.5 |
May–Aug |
|
obovatum |
P |
± taproot |
8–16 |
singly or 2–3/array |
1–6 |
10–13 |
2.5–4 |
0.4–1.2 |
June–July |
*A: annual, P: perennial, S: shrub
Acknowledgements
Funding for Western Forbs: Biology, Ecology, and Use in Restoration was provided by the USDI BLM Great Basin Native Plant Materials Ecoregional Program through the Great Basin Fire Science Exchange. Great thanks to the chapter reviewers: Scott H. Harris, Institute for Applied Ecology, and Amy Bartow, USDA Natural Resources Conservation Service.
This research was supported in part by the USDA Forest Service, Rocky Mountain Research Station. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA or U.S. Government determination or policy.
Literature Cited
Alexander, E.B. 1988. Morphology, fertility, and classification of productive soils on serpentinized peridotite in California (U.S.A.). Geoderma. 41(3): 337-351.
Applestein, C.; Bakker, J.D.; Delvin, E.G.; Hamman, S.T. 2018. Evaluating seeding methods and rates for prairie restoration. Natural Areas Journal. 38(5): 347-355.
Archibald, C. 2006. Seed production protocols for Anaphalis margaritacea, Eriophyllum lanatum, and Eriogonum umbellatum. Native Plants Journal. 7(1): 47-51.
Association of Official Seed Analysts [AOSA]. 2016. AOSA rules for testing seeds. Vol. 1. Principles and procedures. Washington, DC: Association of Official Seed Analysts.
Atzet, T.; White, D.E.; McCrimmon, L.A.; Martinez, P.A.; Fong, P.R.; Randall, V.D., tech. coords. 1996. Field guide to the forested plant associations of southwestern Oregon. Portland, OR: Tech. Pap. R6-NR-ECOL-TP-17-96. U.S. Department of Agriculture, Forest Service, Pacific Northwest Region. 372 p.
Barner, J. 2009a. Propagation protocol for production of propagules Eriophyllum lanatum (Pursh) Forbes seeds (small seed lot). Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://npn.rngr.net/propagation/protocols [Accessed 2021 May 6].
Bartow, A. 2015. Native seed production manual for the Pacific Northwest. Corvallis, OR: U.S. Department of Agriculture, Natural Resources Conservation Service, Corvallis Plant Materials Center. 191 p.
Bartow, A. 2018a. Upland prairie WHIP planting, Polk County, Oregon. Corvallis, OR: U.S. Department of Agriculture, Natural Resources Conservation Service, Corvallis Plant Materials Center. 4 p.
Bartow, A. 2018b. Pollinator habitat in vineyard rows, Polk County, Oregon: Field planting case study report. Corvallis, OR: U.S. Department of Agriculture, Natural Resources Conservation Service, Corvallis Plant Materials Center. 6 p.
Bartow, A. N.D. Harvest methods in forb seed production fields. Poster. Corvallis, OR: U.S. Department of Agriculture, Natural Resources Conservation Service, Corvallis Plant Materials Center. 1 p.
Basey, A.C.; Fant, J.B.; Kramer, A.T. 2015. Producing native plant materials for restoration: 10 rules to collect and maintain genetic diversity. Native Plants Journal. 16(1): 37-53.
Berch, S.M.; Gamiet, S.; Deom, E. 1988. Mycorrhizal status of some plants of southwestern British Columbia. Canadian Journal of Botany. 66(10): 1924-1928.
Blackwell, L.R. 2006. Great Basin wildflowers: A guide to common wildflowers of the high deserts of Nevada, Utah, and Oregon. Helena, MT: Morris Book Publishing. 288 p.
Blakeley-Smith, M.R. 2006. Performance of Willamette Valley native plants following herbicide exposure. Corvallis, OR: Oregon State University. Thesis. 73 p.
Bohlman, G.N.; North, M.; Safford, H.D. 2016. Shrub removal in reforested post-fire areas increases native plant species richness. Forest Ecology and Management. 374: 195-210.
Bower, A.D.; St. Clair, J.B.; Erickson, V. 2014. Generalized provisional seed zones for native plants. Ecological Applications. 24(5): 913-919.
Boyer, L. 2008. Providing native plant diversity to the Willamette Valley ecoregion: No-tech, low-tech, and old-tech seed production methods. Native Plants Journal. 9(3): 230-240.
Cane, J.H.; Love, B.; Swoboda, K. 2012. Breeding biology and bee guild of Douglas’ dustymaiden, Chaenactis douglasii (Asteraceae, Helenieae). Western North American Naturalist. 72(4): 563-568.
Carlquist, S. 1956. On the generic limits of Eriophyllum (Compositae) and related genera. Madroño. 13(7): 226-239.
Carr, C.A.; Krueger, W.C. 2012. The role of the seed bank in recovery of understory species in an eastern Oregon ponderosa pine forest. Northwest Science. 86(3): 168-178.
Chambers, K.L. 1974. Notes on the flora of Clatsop County, Oregon. Madroño. 22(5): 278-279.
Clark, D.L. 2002. Controlling woody vegetation in wetland prairies, 1994-2001. Project No. HE-R01-0378. submitted to Coast Range Resource Area Bureau of Land Management, Eugene, OR. 39 p.
Clark, D.L.; Wilson, M.V. 2001. Fire, mowing, and hand-removal of woody species in restoring a native wetland prairie in the Willamette Valley of Oregon. Wetlands. 21(1): 135-144.
Clark, D.L.; Wilson, M.V. 2005. Restoration of native upland prairies: Habitat for Fenders blue butterfly (Icaricia icarioides fenderi). Order No. 10181M660 prepared for Oregon Fish and Wildlife Service and US Fish and Wildlife Service, Portland, OR. 30 p.
Consortium of Pacific Northwest Herbaria [CPNWH]. 2020. Seattle, WA: University of Washington Herbarium, Burke Museum of Natural History and Culture. http://www.pnwherbaria.org/index.php
Craighead, J.J.; Craighead, F.C. Jr.; Davis, R.J. 1963. A field guide to Rocky Mountain wildflowers from northern Arizona and New Mexico to British Columbia. Boston, MA: Houghton Mifflin Company. 277 p.
del Moral, R. 1984. The impact of the Olympic marmot on subalpine vegetation structure. American Journal of Botany. 71(9): 1228-1236.
Diaz, N.A. 2020. Recurrent formation, low levels of ecological differentiation, and secondary dispersal facilitate the establishment and persistence of autopolyploids in Eriophyllum lanatum. Portland, OR: Portland State University. 51 p.
Dorn, R.D.; Dorn, J.L. 2007. Growing native plants of the Rocky Mountain area. Morrisville, NC: Lulu.com. 260 p.
Drake, D.; Ewing, K. nd. Germination requirements of 32 native Washington prairie species. Seattle, WA: The Nature Conservancy: 181-190.
Driscoll, R.S. 1964. Vegetation-soil units in the central Oregon juniper zone. Res. Pap. PNW-19. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 60 p.
Dyrness, C.T.; Youngberg, C.T. 1966. Soil-vegetation relationships within the ponderosa pine type in the central Oregon pumice region. Ecology. 47(1): 122-138.
European Native Seed Conservation Network [ENSCONET]. 2009. ENSCONET seed collecting manual for wild species. Edition 1: 32 p.
Everett, P. 1957. A summary of the culture of California plants at the Rancho Santa Ana Botanic Garden 1927-1950. Claremont, CA: The Rancho Santa Ana Botanic Garden. 223 p.
Ewing, K. 2002. Mounding as a technique for restoration of prairie on a capped landfill in the Puget Sound lowlands. Restoration Ecology. 10(2): 289-296.
Farr, D.F.; Rossman, A.Y. 2017. Fungal databases, U.S. National Fungus Collections. U.S. Department of Agriculture, Agricultural Research Service. https://nt.ars-grin.gov/fungaldatabases/.
Fertig, W.F.; Massatti, R.T.; Nelson, B.E.; Hartman, R.L. 2013. Annotated checklist of the vascular flora of the Wind River Range, Wyoming (U.S.A.). Journal of the Botanical Research Institute of Texas. 7(2): 905-939.
Franklin, J.F.; Dyrness, C.T. 1973. Natural vegetation of Oregon and Washington. Gen. Tech. Rep. PNW-8. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. 452 p.
Hendricks, L. 2016. The performance of four native perennial forb species along a climate gradient in Pacific Northwest prairies. Eugene, OR: University of Oregon. Thesis. 95 p.
Hickman, J.C., ed. 1993. The Jepson manual: Higher plants of California. Berkeley, CA: University of California Press. 1400 p.
Hitchcock, C.L.; Cronquist, A. 2018. Flora of the Pacific Northwest: An illustrated manual. Second Ed. Giblin, D.E.; Legler, B.S.; Zika, P.F.; Olmstead, R.G., eds. Seattle, WA: University of Washington Press. 882 p.
Horner, M.A. 2001. Vascular flora of the Glass Mountain region, Mono County, California. Aliso. 20(2): 75-105.
Ingham, E.R.; Wilson, M.V. 1999. The mycorrhizal colonization of six wetland plant species at sites differing in land use history. Mycorrhiza. 9(4): 233-235.
ITIS Database. 2021. Integrated Taxonomic Information System. Available: http://www.itis.gov/index.html
James, J.J.; Drenovsky, R.E. 2007. A basis for relative growth rate differences between native and invasive forb seedlings. Rangeland Ecology and Management. 60(4): 395-400.
Jensen, A.S.; Barjadze, S.; Kanturski, M. 2020. A review of the aphid genus Macrosiphoniella del Guercio, 1911 (Hemiptera: Aphididae) in the USA with description of a new species. European Zoological Journal. 87(1): 412-443.
Johnson, C.G.; Swanson, D.K. 2005. Bunchgrass plant communities of the Blue and Ochoco Mountains: A guide for managers. Gen. Tech. Rep. PNW-GTR-641. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 119 p.
Johnson, D.E.; Mooring, J.S. 2006. Eriophyllum. In: Flora of North America Editorial Committee, ed. Flora of North America North of Mexico. Volume 21 Magnoliophyta: Asteridae, Part 8: Asteraceae, Part 3. New York, NY: Oxford University Press: 353-362.
Kesonie, D.T.; Hartman, R.L. 2011. A floristic inventory of Grand Teton National Park, Pinyon Peak Highlands, and vicinity, Wyoming, U.S.A. Journal of the Botanical Research Institute of Texas. 5(1): 357-388.
Kuramoto, R.T.; Bliss, L.C. 1970. Ecology of subalpine meadows in the Olympic Mountains, Washington. Ecological Monographs. 40(3): 317-347.
LaBar, C.C.; Schultz, C.B. 2012. Investigating the role of herbicides in controlling invasive grasses in prairie habitats: Effects on non-target butterflies. Natural Areas Journal. 32(2): 177-189.
Lady Bird Johnson Wildflower Center [LBJWC]. 2016. Eriophyllum lanatum (Pursh) Forbes. Native Plant Database. Austin, TX: Lady Bird Johnson Wildflower Center. https://www.wildflower.org/plants-main [Accessed 2021 May 6].
Lambert, S. 2005. Guidebook to the seeds of native and non-native grasses, forbs and shrubs of the Great Basin. Boise, ID: U.S. Department of the Interior, Bureau of Land Management, Idaho State Office. 136 p.
Landis, T.D.; Wilkinson, K.M.; Steinfeld, D.E.; Riley, S.A.; Fekaris, G.N. 2005. Roadside revegetation of forest highways: New applications for native plants. Native Plants Journal. 6(3): 297-305.
Lavin, M. 1983. Floristics of the upper Walker River, California and Nevada. The Great Basin Naturalist. 43(1): 93-130.
Lawrence, B.A.; Kaye, T.N. 2008. Direct and indirect effects of host plants: Implications for reintroduction of an endangered hemiparasitic plant (Castilleja levisecta). Madroño. 55(2): 151-158.
Leigh, M.; Pushnik, J.C.; Boul, R.D.; Brown, M.R.; Hunt, J.W.; Koenig, D.A. 2006. Propagation protocol for production of container (plug) Eriophyllum lanatum (Pursh) Forbes plants. Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://npn.rngr.net/propagation/protocols [Accessed 2021 May 6].
Luna, T.; Mousseaux, M.R.; Dumroese, R.K. 2018. Common native forbs of the northern Great Basin important for greater sage-grouse. Gen. Tech. Rep. RMRS-GTR-387. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station; Portland, OR: U.S. Department of the Interior, Bureau of Land Management, Oregon-Washington Region. 76 p.
MacDougall, A.S.; Turkington, R. 2006. Dispersal, competition, and shifting patterns of diversity in a degraded oak savanna. Ecology. 87(7): 1831-1843.
Maret, M.P.; Wilson, M.V. 2000. Fire and seedling population dynamics in western Oregon prairies. Journal of Vegetation Science. 11(2): 307-314.
Maret, M.P.; Wilson, M.V. 2005. Fire and litter effects on seedling establishment in western Oregon upland prairies. Restoration Ecology. 13(3): 562-568.
Miller, S.A.; Bartow, A.; Gisler, M.; Ward, K.; Young, A.S.; Kaye, T.N. 2011. Can an ecoregion serve as a seed transfer zone? Evidence from a common garden study with five native species. Restoration Ecology. 19(201): 268-276.
Mitchell, R.M.; Bakker, J.D. 2016. Grass abundance shapes trait distributions of forbs in an experimental grassland. Journal of Vegetation Science. 27(3): 557-567.
Moerman, D. 2003. Native American ethnobotany: A database of foods, drugs, dyes, and fibers of Native American peoples, derived from plants. Dearborn, MI: University of Michigan. http://naeb.brit.org
Mohan, S.K.; Shock, C.C. 2014. Etiology, epidemiology, and management of diseases of native wildflower seed production. In: Kilkenny, F.; Shaw, N.L.; Gucker, C.L., eds. Great Basin Native Plant Project: 2013 Progress Report. Boise, ID: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 107-108.
Mooring, J.S. 1975. A cytogeographic study of Eriophyllum lanatum (Compositae, Helenieae). American Journal of Botany. 62(10): 1027-1037.
Mooring, J.S. 2001. Barriers to interbreeding in the Eriophyllum lanatum (Asteraceae, Helenieae) species complex. American Journal of Botany. 88(2): 285-312.
Mooring, J.S. 2008. An Eriophyllum lanatum (Asteraceae) hybrid zone in Oregon. Madroño. 52(1): 269-279.
Munz, P.A.; Keck, D.D. 1973. A California flora and supplement. Berkeley, CA: University of California Press. 1905 p.
Nagorsen, D.W. 1987. Marmota vancouverensis. Mammalian Species. 270: 1-5.
National Wildlife Federation [NWF]. 2021. Native plant finder. https://www.nwf.org/NativePlantFinder/Plants [Accessed 2021 August 8].
Nyamai, P.A.; Prather, T.S.; Wallace, J.M. 2011. Evaluating restoration methods across a range of plant communities dominated by invasive annual grasses to native perennial grasses. Invasive Plant Science and Management. 4(3): 306-316.
Ogle, D.; St. John, L.; Stannard, M.; Holzworth, L. 2014. Conservation plant species for the Intermountain West. Tech. Note 24. Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 72 p.
Ogle, D.; Tilley, D.; Cane, J.; St. John, L.; Fullen, K.; Stannard, M.; Pavek, P. 2011. Plants for pollinators in the Intermountain West. Plant Materials Tech. Note 2A. Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 40 p.
Omernik, J.M. 1987. Ecoregions of the conterminous United States. Map (scale 1:7,500,000). Annals of the Association of American Geographers. 77(1): 118-125.
Page, J.E.; Huffman, M.A.; Smith, V.; Towers, G.H.N. 1997. Chemical basis for Aspilia leaf-swallowing by chimpanzees: A reanalysis. Journal of Chemical Ecology. 23(9): 2211-2226.
Parkinson, H. 2003. Landscaping with native plants of the Intermountain Region. Tech. Ref. 1730-3. Boise, ID: U.S. Department of the Interior, Bureau of Land Management. 46 p.
Pavek, P.; Erhardt, B.; Heekin, T.; Old, R. 2012. Forb seedling identification guide for the Inland Northwest: Native, introduced, invasive, and noxious species. Pullman, WA: U.S. Department of Agriculture, Natural Resources Conservation Service, Pullman Plant Materials Center. 144 p.
Pavek, P.; Fleenor, R.; Stannard, M.; Dring, T.; Cane, J.; St. John, L.; Tilley, D. 2013. Plants for pollinators in the Inland Northwest (revised). Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service, Pullman Plant Materials Center. 62 p.
Pavek, P.; Jensen, W.; Jensen, J. 2016. Establishing native forbs in existing CRP using no-till techniques in northern Idaho: Comparison of drills and seedbed preparations. Pullman, WA: U.S. Department of Agriculture, Natural Resources Conservation Service, Pullman Plant Materials Center. 20 p.
Pavek, P.L.S. 2011. Plant guide for common woolly sunflower (Eriophyllum lanatum). Pullman, WA: U.S. Department of Agriculture, Natural Resources Conservation Service, Pullman Plant Materials Center. 2 p.
Pendergrass, K.L. 1996. Vegetation composition and response to fire of native Willamette Valley wetland prairies. Corvallis, OR: Oregon State University. Thesis. 241 p.
Peter, D.; Shebitz, D. 2006. Historic anthropogenically maintained bear grass savannas of the southeastern Olympic Peninsula. Restoration Ecology. 14(4): 605-615.
Plant Conservation Alliance [PCA]. 2015. National seed strategy for rehabilitation and restoration 2015-2020. Washington, DC: U.S. Department of the Interior, Bureau of Land Management. 52 p.
Richardson, B.; Kilkenny, F.; St. Clair, B.; Stevenson-Molnar, N. 2020. Climate Smart Restoration Tool. https://climaterestorationtool.org/csrt/
Riegel, G.M.; Franklin, J.F. 1992. Foothill oak woodlands of the interior valleys southwestern Oregon. Northwest Science. 66(2): 66-76.
Riegel, G.M.; Thornburgh, D.A.; Sawyer, J.O. 1990. Forest habitat types of the South Warner Mountains, Modoc County, California. Madrono. 37(2): 88-112.
Rodríguez-Rojo, M.J.; Sánchez-Mata, D.; Gavilán, R.G.; Rivas-Martínez, S.; Barbour, M.G. 2001. Typology and ecology of Californian serpentine annual grasslands. Journal of Vegetation Science. 12(5): 687-698.
Rogers, L.E.; Gano, K.A. 1980. Townsend ground squirrel diets in the shrub-steppe of southcentral Washington. Journal of Range Management. 33(6): 463-465.
Royal Botanic Gardens Kew [RBG Kew]. 2020. Seed Information Database (SID). Version 7.1. http://data.kew.org/sid/ [Accessed 2020 February 16].
Russell, M. 2011. Dormancy and germination pre-treatments in Willamette Valley native plants. Northwest Science. 85(2): 389-402.
Russell, M.C. 2010. Germination and establishment in Willamette Valley native prairie plants. Corvallis, OR: Oregon State University. Thesis. 76 p.
Sampson, A.W. 1944. Plant succession on burned chaparral lands in northern California. Bull. 685. Berkeley, CA: University of California, College of Agriculture, Agricultural Experiment Station. 144 p.
Savage, W. 1973. Annotated checklist of vascular plants of Sagehen Creek Drainage Basin, Nevada County, California. Madroño. 22(3): 115-139.
Schroll, E.; Lambrinos, J.G.; Sandrock, D. 2011. An evaluation of plant selections and irrigation requirements for extensive green roofs in the Pacific Northwestern United States. HortTechnology. 21(3): 314-322.
Schuh, R.T. 2001. Revision of new world Plagiognathus Fieber, with comments on the Palearctic fauna and the description of a new genus (Heteroptera: Miridae: Phylinae). Bulletin of the American Museum of Natural History. 266: 1-264.
Schultz, C.B. 2001. Restoring resources for an endangered butterfly. Journal of Applied Ecology. 38(5): 1007-1019.
Schultz, C.B.; Katrina, M.D. 1999. Nectar and hostplant scarcity limit populations of an endangered Oregon butterfly. Oecologia. 119(2): 231-238.
SEINet – Regional Networks of North American Herbaria Steering Committee [SEINet]. 2020. SEINet Regional Networks of North American Herbaria. https://Symbiota.org/docs/seinet
Shiflet, T.N. 1994. Rangeland cover types of the United States. Denver, CO: Society for Range Management. 152 p.
Shock, C.C.; Feibert, E.B.; Saunders, L.D.; Shaw, N.; Sampangi, R.K. 2014. Seed production of Great Basin native forbs. In: Kilkenny, F; Shaw, N.; Gucker, C., eds. Great Basin Native Plant Project: 2013 Progress Report. Boise, ID: U.S. Department of Agriculture, Rocky Mountain Research Station: 109-155.
Skinner, D.M. 2007. Propagation protocol for production of propagules Eriophyllum lanatum (Pursh) Forbes plants 10 cu. in. Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://npn.rngr.net/propagation/protocols [Accessed 2021 May 6].
Spellenberg, R. 2001. National Audubon Society field guide to North American wildflowers: Western region, revised edition. New York, NY: Alfred A. Knopf, Inc. 862 p.
Spence, J.R.; Shaw, R.J. 1981. A checklist of the alpine vascular flora of the Teton Range, Wyoming, with notes on biology and habitat preferences. The Great Basin Naturalist. 41(2): 232-242.
Stuart, J.D.; Worley, T.; Buell, A.C. 1996. Plant associations of Castle Crags State Park, Shasta County, California. Madroño. 43(2): 273-291.
Taylor, R.J. 1992. Sagebrush country: A wildflower sanctuary. Missoula, MT: Mountain Press Publishing Company. 211 p.
Thomas, T.; Gamon, J. 1997. Restoration of a prairie plant community: Help for a threatened species. In: Warwick, C., ed. Fifteenth North American Prairie Conference Proceedings; 1997; St. Charles, IL. Bend, OR: Natural Areas Association: 244-248.
Thompson, R.L.; Skeese, J.R. 2005. Vascular flora of Golden and Silver Falls State Natural Area in the Oregon Coast Range, Coos County, Oregon. Madroño. 52(4): 215-221.
Titus, J.H.; Moore, S.; Arnot, M.; Titus, P.J. 1998. Inventory of the vascular flora of the blast zone, Mount St. Helens, Washington. Madroño. 45(2): 146-161.
Tveten, R.K.; Fonda, R.W. 1999. Fire effects on prairies and oak woodlands on Fort Lewis, Washington. Northwest Science. 73(3): 145-158.
University of Wyoming Extension [WY Extension]. 2021. Native plants for the Intermountain West. Laramie, WY: University of Wyoming. https://cwel.usu.edu/westernnativeplants/index.php.
USDA Forest Service, Bend Seed Extractory [USDA FS BSE]. 2017. Nursery Management Information System Version 4.1.11. Local Source Report 34-Source Received. Bend, OR: U.S. Department of Agriculture, Forest Service, Bend Seed Extractory.
USDA Forest Service, Western Wildland Environmental Threat Assessment Center [USFS WWETAC]. 2017. TRM Seed Zone Applications. Prineville, OR: U.S. Department of Agriculture, Forest Service, Western Wildland Environmental Threat Assessment Center. https://www.fs.fed.us/wwetac/threat-map/TRMSeedZoneMapper.php
USDA Natural Resources Conservation Service [USDA NRCS]. 2021. The PLANTS Database. Greensboro, NC: U.S. Department of Agriculture, Natural Resources Conservation Service, National Plant Data Team. https://plants.usda.gov/java
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2016. Bureau of Land Management technical protocol for the collection, study, and conservation of seeds from native plant species for Seeds of Success. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program. 37 p.
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2017. Seeds of Success collection data. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program.
USDI Environmental Protection Agency [USDI EPA]. 2017. Ecoregions. Washington, DC: U.S. Department of the Interior, Environmental Protection Agency. https://www.epa.gov/eco-research/ecoregions
USDI Geological Survey [USDI USGS]. 2020. Biodiversity Information Serving Our Nation (BISON). U.S. Geological Survey. https://www.gbif.us/#home
Utah Crop Improvement Association [UCIA]. 2015. How to be a seed connoisseur. Logan, UT: UCIA, Utah Department of Agriculture and Food, Utah State University and Utah State Seed Laboratory. 16 p.
Vance, N.; Neill, A.; Morton, F. 2006. Native grass seeding and forb planting establishment in a degraded oak savanna plant community in the Coast Range foothills of western Oregon. Native Plants Journal. 7(1): 35-46.
Walker, R.B. 1954. The ecology of serpentine soils: II. Factors affecting plant growth on serpentine soils. Ecology. 35(2): 259-266.
Wall, M.; MacDonald, J. 2009. Processing seeds of California native plants for conservation, storage, and restoration. Claremont, CA: Rancho Santa Ana Botanic Garden. 216 p.
Ward, K.; Gisler, M.; Fiegener, R.; Young, A. 2008. The Willamette Valley seed increase program: Developing genetically diverse germplasm using an ecoregion approach. Native Plants Journal. 9(3): 334-350.
Waring, R.H.; Major, J. 1964. Some vegetation of the California coastal redwood region in relation to gradients of moisture, nutrients, light, and temperature. Ecological Monographs. 34(2): 167-215.
Waters, S.M.; Fisher, S.E.; Lambers, J.H.R. 2014. Neighborhood-contingent indirect interactions between native and exotic plants: Multiple shared pollinators mediate reproductive success during invasions. Oikos. 123(4): 433-440.
Welsh, S.L.; Atwood, N.D.; Goodrich, S.; Higgins, L.C., eds. 2015. A Utah Flora. Fifth Edition, revised. Provo, UT: Brigham Young University. 990 p.
Whittaker, R.H. 1960. Vegetation of the Siskiyou Mountains, Oregon and California. Ecological Monographs. 30(3): 279-338.
Wilson, M.V.; Ingersoll, C.A.; Wilson, M.G.; Clark, D.L. 2004. Why pest plant control and native plant establishment failed: A restoration autopsy. Natural Areas Journal. 24(1): 23-31.
Wyniger, D. 2011. Revision of the Nearctic genus Coquillettia uhler with a transfer to the tribe Phylini, the description of 14 new species, a new synonymy, and the description of two new Nearctic genera Leutiola and Ticua and two new species (Heterophtera: Miridae: Phylinae). Entomologica Americana 117(3): 134-211.
Young, S.A.; Schrumpf, B.; Amberson, E. 2003. The Association of Official Seed Certifying Agencies (AOSCA) native plant connection. Moline, IL: AOSCA. 9 p. Available: https://seedcert.oregonstate.edu/sites/seedcert.oregonstate.edu/files/pdfs/aoscanativeplantbrochure.pdf
Youngblood, A.; Metlen, K.L.; Coe, K. 2006. Changes in stand structure and composition after restoration treatments in low elevation dry forests of northeastern Oregon. Forest Ecology and Management. 234(1): 143-163.
Youtie, B.A. 1992. Biscuit scabland restoration includes propagation studies. Restoration and Management Notes. 10(1): 79-80.
How to Cite
Gucker, Corey L.; Shaw, Nancy L. 2021. Common woolly sunflower (Eriophyllum lanatum). In: Gucker, C.L.; Shaw, N.L., eds. Western forbs: Biology, ecology, and use in restoration. Reno, NV: Great Basin Fire Science Exchange. Online: https://westernforbs.org/species/common-woolly-sunflower-eriophyllum-lanatum/