Authorship
Gucker, Corey; Shaw, Nancy
Publication Date
February 2018
Update
February 2024
Nomenclature
Chaenactis douglasii (Hook.) Hook. & Arn., hereafter referred to as Douglas’ dustymaiden, belongs to the Heliantheae tribe of the Asteraceae family (Hickman 1993; Pavek et al. 2012).
Family
Asteraceae – Aster family
Genus
Chaenactis
Species
douglasii
NRCS Plant Code
CHDO (USDA NRCS 2024).
Subtaxa
The Flora of North America (Morefield 2006) recognizes two varieties of Douglas’ dustymaiden: Chaenactis douglasii var. douglasii and var. alpina (A. Gray). The Intermountain Flora (Cronquist 1994) describes five varieties, but these are primarily ecogeographic variants and distinguishing characteristics are ill-defined.
Synonyms
Chaenactis douglasii: Hymenopappus douglasii Hook., Macrocarphus douglasii (Hook.) Nutt. (Morefield 2006).
C. d. var. douglasii: Chaenactis angustifolia Greene, C. douglassii var. achilleifolia (Hook. & Arnott) A. Gray, C. d. var. montana M.E. Jones, C. d. var. rubricaulis (Ryd.) Ferris, C. pedicularia Greene, C. pumila Greene, C. ramosa Stockwell (Morefield 2006).
C. d. var. alpina: C. alpina (A. Gray) M.E. Jones, C. alpina var. leucopsis (Greene) Stockwell, C. alpina var. rubella (Greene) Stockwell, C. panamintensis Stockwell (Morefield 2006).
Common Names
Douglas’ dustymaiden, bride’s bouquet, Douglas pincushion, dusty-maiden, duskymaiden, hoary pincushion, false yarrow (Craighead et al. 1963; Anderson and Holmgren 1996; Lambert 2005; Morefield 2006; Tilley et al. 2010; USDA NRCS 2024). Alpine dustymaiden and pincushion are common names for C. d. var. alpina (Morefield 2006).
Chromosome Number
Douglas’ dustymaiden chromosome numbers are highly variable with 2n = 6, 12, 13, 14, 15, 16, 17, 18, 24 and 36 possible (Mooring 1980; Cronquist 1994), but chromosome numbers of 2n = 12, 24, and 36 predominate (Morefield 2006). Plants with different ploidy levels can co-occur without any obvious distinction (Cronquist 1994).
When 512 plants from 200 populations were analyzed, tetraploids were most frequent (47.2%), followed by diploids (35.8%) and hexaploids (7.4%). For each population, plants were almost exclusively diploid, tetraploid, or hexaploid (Mooring 1980). Tetraploids and hexaploids are typically winter annuals or biennials found in the Columbia Plateau and Great Basin provinces (Mooring 1992). Diploids are perennials and found in certain deep river canyons or gorges of the Intermountain Region. In this region, ploidy level is associated with geologic substrate age rather than climate, elevation, vegetation, or soil type. Diploids predominate on geologically older substrates of mesic montane and riparian sites. Polyploids predominate on younger volcanic and alluvial substrates of more arid environments (Mooring 1992).
Hybridization
Varieties alpina and douglasii intergrade where their distributions overlap (Hickman 1993).
Distribution
Douglas’ dustymaiden is native to North America and is widely distributed throughout the West, occurring from southern Alberta, British Columbia, and Saskatchewan to California, Nevada, northern Arizona, northern New Mexico, Colorado, and the Dakotas (Cronquist 1994; Morefield 2006; LBJWC 2017). Variety douglasii is more widespread, occurring throughout the range reported for the species. Variety alpina occurs only in Montana, Idaho, Oregon, California, Utah, Colorado, and Wyoming (Morefield 2006).
Habitat And Plant Associations
Douglas’ dustymaiden commonly occupies dry, open sites with sandy or gravelly soil (Fig. 1) (Cronquist 1994; Lambert 2005) where annual precipitation averages 10 in (250 mm) or more (Ogle et al. 2012). It occurs in a variety of plant communities from lowland valleys to timberline and above, including grasslands, shadscale (Atriplex confertifolia), desert saltbush (A. polycarpa), sagebrush (Artemisia spp.), pinyon and juniper (Pinus–Juniperus spp.), and conifer-dominated plant communities (Hatton and West 1987; Pavek et al. 2012; Welsh et al. 2015).
Douglas’ dustymaiden is often an early colonizer of disturbed and harsh sites. It grows in cinder garden communities at Craters of the Moon National Monument in central Idaho where the basalt parent material is young and growing conditions are extremely harsh (Day and Wright 1985).
Increasing summer precipitation and decreasing annual minimum temperature had a negative effect on the potential climate suitability for Douglas’ dustymaiden in a study that coupled herbarium records and ecological niche modelling to describe the suitable climates for Great Basin forb species. Douglas’ dustymaiden was associated with sites with relatively low minimum temperatures and high year-to-year variation in minimum temperatures (Barga et al. 2018).
Elevation
Douglas’ dustymaiden’s distribution ranges from near sea level to about 13,100 ft (4,000 m) (Mooring 1992; Morefield 2006). Variety douglasii occupies a broader elevation range and occurs at lower elevations than variety alpina, which is generally found between 8,800 and 13,100 ft (2,700-4,000 m) (Morefield 2006). In California, variety douglasii occurs at elevations between 3,200 and 11,500 ft (1,000-3,500 m), and variety alpina occurs at elevations of 9,800 to 11,500 ft (3,000–3,500 m) (Hickman 1993). In Utah, the species is found at elevations of 4,400 to 10,000 ft (1,340-3,050 m) (Welsh et al. 2015) and in Oregon, at elevations of 0 to 7,550 ft (2,300 m) (Chambers 2020).
Soils
Douglas’ dustymaiden occupies numerous substrates ranging from dry to medium moist (Craighead et al. 1963; Welsh et al. 2015). Gravelly clays, silts, or sands were the common soil types at Douglas’ dustymaiden seed collection sites throughout the West (USDI BLM SOS 2017). In Yellowstone National Park it is often found on dry, coarse, exposed soils, especially obsidian sands (Link 1993). Reports suggest that Douglas’ dustymaiden has low calcium carbonate tolerance and uses little water (LBJWC 2017), although it has grown well in common gardens with alkaline calcareous soils (J. Cane, USDA ARS, personal communication, August 2017).
On the Wind River Indian Reservation in Wyoming, Douglas’ dustymaiden was common in slender wheatgrass (Elymus trachycaulus subsp. trachycaulus)-forb communities at 10,400 ft (3,180 m) where soils were 57% sand, 23% silt, and 20% clay with 4% organic matter and a pH of 7.6 (Friday and Scasta 2020).
Douglas’ dustymaiden was common on silver mine dumps near Park City, Utah, visited about 45 years after the end of surface mining operations (Alvarez et al. 1974). It occurred on sites where mid-July soil temperatures averaged 62.2° F (16.8 °C) at 15 in (38 cm) deep, phosphorous levels averaged 784 µg/300 cc of soil, and calcium levels averaged 376 mg/300 cc of soil. Density of the species increased significantly (P = 0.01) with increasing soil phosphorus (Alvarez et al. 1974). On sulfide-bearing waste areas left from copper pit mining operations at the Bingham Canyon Mine in Utah, Douglas’ dustymaiden occurred on both saline (0.06–0.5 dS/m) and low to neutral pH (4.2-7.9) soils (Borden and Black 2005).

Figure 1. Douglas’ dustymaiden in natural rangeland habitat. Photo: USFS.
Description
Douglas’ dustymaiden is morphologically variable (Morefield 2006; Hitchcock and Cronquist 2018). It grows as a annual, biennial, or short-lived perennial and in rare cases can be slightly woody (Hickman 1993; Cronquist 1994). Variety douglasii is typically a short-lived perennial (Cronquist 1994).
Plants are taprooted with a simple to tightly branched and well developed caudex (Welsh et al. 2015; Hitchcock and Cronquist 2018). The root systems of two plants growing on the southern slope of Long Gulch in the Boise River Watershed in Idaho were excavated. These plants had slender taproots extending 6 in (15 cm) deep at which point they divided into a network of fine lateral roots spanning up to 10 in (25 cm) wide and reaching 28 to 35 in (70-90 cm) deep (Woolley 1936).
Plants range from 6 to 36 in (7-91 cm) tall but often are shorter than 24 in (61 cm) (Lambert 2005; Ogle et al. 2011; Pavek et al. 2012). Variety douglasii is generally taller (ranging from 3 to 24 in [8–61 cm]) and grows more erect than variety alpina (typically 2 to 4 in [5-10 cm] tall), which has a caespitose to matted growth habit (Cronquist 1994; Morefield 2006). Stems of Douglas’ dustymaiden are solitary to many (25 or more) and erect to spreading with sparse to dense cobwebby hairs that tend to fade upward and with age (Cronquist 1994; Luna et al. 2018).
Plants have alternate, finely dissected, gray-green leaves. Leaves are strictly basal for variety alpina, but basal and stem leaves are common for variety douglasii (Welsh et al. 2015). Leaves are petiolate and tomentose with small white hairs. Leaves are progressively smaller and less dissected up the stem and hairiness decreases with plant age. Leaves range from 0.8 to 5 in (2-12 cm) long, and the largest leaves are typically twice pinnately lobed with the ultimate lobes inrolled (Anderson and Holmgren 1996; Hickman 1993; Morefield 2006; Pavek et al. 2012; Welsh et al. 2015; Hitchcock and Cronquist 2018; Luna et al. 2018; Chambers 2020).
Individual flowers are tiny, tubular, bisexual, pinkish to white, and number 10 to 50 in a leafy, flat-topped inflorescence (cyme) less than 1 in (2.5 cm) wide and tall (Fig. 2) (Hickman 1993; Anderson and Holmgren 1996; Pavek et al. 2012; Hitchcock and Cronquist 2018; Chambers 2020). Variety douglasii produces 1 to 25 or more flower heads per stem, but variety alpina produces only one or two (Morefield 2006; Welsh et al. 2015; Hitchcock and Cronquist 2018). Individual flowers are radially symmetrical disk flowers near the center of the head with ray flowers around the edge, although all flowers in one head may be disk or ray flowers (LBJWC 2017). Involucres measure 8 to 12 mm high with phyllaries in two to five unequal series with 13 to 25 bracts (Cronquist 1994; Morefield 2006; Welsh et al. 2015). Fruits are stiffly hairy, club-shaped achenes (cypselas) measuring 5 to 8 mm long (Hickman 1993) with an apical pappus with unequal scales in three to four series (Chambers 2020). Mature fruit color ranges from gold to black. Seed is readily wind dispersed (Tilley et al. 2010) (Fig. 3).
Reproduction
Douglas’ dustymaiden reproduces entirely from seed. Flowering is indeterminate and occurs in the first year (Shock et al. 2014c).

Figure 2. Douglas’ dustymaiden flowers. Photo: J. Cane, USDA ARS.

Figure 3. Douglas’ dustymaiden achene with pappus. Photo: J. Cane, USDA ARS.
Phenology
Douglas’ dustymaiden flowers appear from spring through summer (April to September) (Cronquist 1994; Parkinson and DeBolt 2005; Chambers 2020), but June and July are most typical (Andersen and Homgren 1996; LBJWC 2017). Variety douglasii flowers from May to September and variety alpina from July to September (Morefield 2006).
Based on 2 years of observations during a time when moisture and temperatures were near the 30-year average in ponderosa pine (Pinus ponderosa)/big sagebrush communities near Penticton, British Columbia, Douglas’ dustymaiden initiated growth in March and April, began flowering in April and May, reached full flower in May, produced and shattered seed in June, and cured in July (Pitt and Wikeem 1990).
Breeding System
Although Douglas’ dustymaiden can produce seed through selfing, cross pollination by insects results in the greatest seed production (Cane et al. 2012).
Pollination
In experiments conducted at the U.S. Department of Agriculture’s Pollinating Insect Research Unit in Logan, Utah, cross-pollinated flowers produced four times more filled achenes than self-pollinated flowers (P < 0.05) (Cane et al. 2012). Only bees were found visiting Douglas’ dustymaiden flowers in wild and cultivated stands. Visitors were sparse, averaging 1 bee/plant, but the fauna from the limited collections was rich with 19 species represented. Bee collections suggested that nonsocial univoltine native bees were the primary pollinators. Most of the bees were floral generalists (Cane et al. 2012). The most practical, manageable bees to use to pollinate crops are the cavity-nesting Asteraceae specialist (Osmia californica) and the European honey bee (Apis mellifera) (Cane 2008; Cane et al. 2012).
Ecology
Douglas’ dustymaiden is a colonizer of early-seral communities (Day and Wright 1985) and often grows on disturbed sites (Craighead et al. 1963; Huffman et al. 2017; Rink et al. 2020), but it also occurs in late-seral communities (Koniak 1985). It may also play a role in advancing successional development. In Lassen Volcanic National Park, Douglas’ dustymaiden grew as a mat and produced dense foliage that captured fine-moving debris and served to build up soil on the talus surface (Pérez 2012).
Seed And Seedling Ecology
Several seed and plant characteristics suggest that Douglas’ dustymaiden opportunistically colonizes disturbed sites and openings in existing communities. These characteristics include reproduction during the first growth season, production of small, easily wind-dispersed fruits, and high establishment for fall-planted seed (Parkinson and DeBolt 2005; Tilley 2011).
Douglas’ dustymaiden may not produce a persistent seed bank based on a study comparing the aboveground and belowground species composition in big sagebrush vegetation in northeastern Nevada (Barga and Leger 2018). Douglas’ dustymaiden occurred in the aboveground vegetation at 5 of 17 plots, but did not emerge from soil samples collected at any of the 17 plots. Soil sampling occurred in June when most seeds had germinated for the season but before current year’s seeds had matured and dispersed. Soil samples were exposed to a wide variety of treatments to encourage germination of dormant seed (Barga and Leger 2018).
Agneray et al. (2022) conducted greenhouse studies that provide some information about seed and seedling relationships for Douglas’ dustymaiden. The study used seed collected throughout the western Great Basin (annual precipitation: 7.5-15.3 in [190-388 mm]; elevation: 4,183-7,874 ft [1,275-2,400 m]) and evaluated a variety of shrub, grass, and forb species. Survival of Douglas’ dustymaiden in the greenhouse averaged 21% and ranged from 3 to 50%. Seed weight was a weak predictor of seedling survival. Higher root mass was associated with increased survival for older seedlings (40 days). The slope of the seed collection site was associated with seedling survival. Seedlings from seed collected at flatter sites had the highest survival in competitive environments (1 native seed with 3 cheatgrass [Bromus tectorum] seeds in 0.16 L pots). For the experiments, pots were kept in a greenhouse with temperatures that mimicked the transition from cooler to warmer seasons that are typical of the Great Basin environment. Media in the pots was a 10:25:65 mix of perlite: sand: coarse-textured field soil collected in Dayton, Nevada (Agneray et al. 2022).
Disturbance Ecology
Several studies suggest that Douglas’ dustymaiden is disturbance tolerant. Plant frequency was nearly the same at treated and untreated sites following lop and scatter woodland thinning in northwestern Arizona (Huffman et al. 2017). The species occurred in each of the first three post-treatment years following an anchor chaining treatment in pinyon-juniper near Ephraim, Utah, but its density and frequency decreased as time since disturbance increased (Davis and Harper 1990).
Douglas’ dustymaiden is sometimes common in early post-fire vegetation. In studies following fall and spring prescribed fires in a mountain big sagebrush (A. tridentata subsp. vaseyana)/bunchgrass habitat at Lava Beds National Monument in northern California, seedlings germinated from soil collected immediately following and 1 year after a fall prescribed burn (Ellsworth and Kauffman 2013; Ellsworth, personal communication, Oregon State University, May 2017 ). Plants were present the first year following a fall prescribed fire in Wyoming big sagebrush (A. t. subsp. wyomingensis) in southeastern Oregon (Wrobleski 1999) and on 1-, 2-, and 3-yr old burned sites in sagebrush-pinyon-juniper vegetation in west-central Utah (Ott et al. 2003). When pinyon-juniper sites burned between 1 and 60 years earlier were evaluated in California and Nevada, Douglas’ dustymaiden frequency was not significantly different in any of the seral stages (Koniak 1985).
Wildlife And Livestock Use
Douglas’ dustymaiden is used by wildlife. In a forb common garden installed in the Glass Buttes region of Oregon, Douglas’ dustymaiden plants were relished by pronghorn (observed) and cattle (suspected) (S. Barga, USFS, personal communication, April 2024). Greater sage grouse (Centrocercus urophasianus) feed on the stems and leaves of Douglas’ dustymaiden (Luna et al. 2018) and the spotted buff gem moth (Heliothis phloxiphaga) uses Douglas’ dustymaiden as a host (Robinson et al. 2023). It also has high nutrient value for native bees and moths (Chambers 2020).
Ethnobotany
Douglas’ dustymaiden was important to Indians throughout western North America. The Gosiute of Utah, Shoshoni of Nevada, and Paiutes of Oregon used the plant to treat body soreness and aches (Chamberlin 1911; Train et al. 1941; Mahar 1953). The Gosiute minced or mashed the plants and applied the paste directly to sore limbs or other body parts to relieve aches (Chamberlin 1911). The Paiutes of Oregon made a tea from young plants that were gathered before flowering. This tea was drunk or put on the hair to treat headaches (Mahar 1953). The Thompson Indians of British Columbia drank a strong tea to alleviate swelling, skin problems, and insect and snake bites (Shemluck 1982). The Paiute and Shoshoni peoples of Nevada referred to Douglas’ dustymaiden as ‘swelling medicine’ (Train et al. 1941). Fresh plants or leaves were crushed and applied as a poultice for soreness or swellings. The plant or leaves were also boiled and drunk to treat coughs, colds, indigestion, or sour stomach. Sometimes plants were heated in a tub of water and used for soaking to treat swellings or edema (Train et al. 1941). Tribes around Elko, Nevada, reported steeping the whole plant to make a tea to slow children’s heart beats (Nickerson 1966). The Okanagan of British Columbia used a plant infusion to treat chapped hands, pimples, boils, tumors, swellings, and insect and snake bites (Perry 1952). Okanagan-Colville Indians of British Columbia and Washington steeped Douglas’ dustymaiden roots in hot water and used the infusion as an eyewash (Turner et al. 1980). The Salishan people of northeastern Washington ingested a decoction of roots to purge or avoid illness (Ray 1933). The Thompson Indians of British Columbia drank a mild tea to treat stomach problems (Shemluck 1982). Some Thompson people cautioned against applying the plant to tumors or external boils (Steedman 1928).
Current Medicinal Use
In an evaluation of the phytochemistry of Douglas’ dustymaiden, the essential oil extracted was low and dominated by tiglic acid and thymol. Thymol may account for reported antimicrobial effects (Poudel et al. 2023).
Horticulture
Douglas’ dustymaiden seed is commercially available for horticultural uses (Morefield 2006). Its clusters of cream to pinkish flowerheads, divided lacy gray-green leaves, and attractiveness to native pollinators makes it a desirable garden plant (Chambers 2020). It grows well in sandy or other well-drained, low fertility soils in USDA hardiness zones 6 to 9 (PFAF 2024). It prefers full sun, making it suitable for rock gardens and other dry border plantings (Chambers 2020; PFAF 2024).
Revegetation Use
Rapid growth (Ogle et al. 2019), seed production in the first growing season (Tilley 2011; Shock et al. 2017a), and attractiveness to pollinators (Day and Wright 1985; Wrobleski 1999; Ott et al. 2003; Cane et al. 2012; Eldredge et al. 2013; Ogle et al. 2019) make Douglas’ dustymaiden a good addition to restoration or rehabilitation seeding mixes. It is a good choice when developing pollinator seed mixtures (Cane et al. 2012; Ogle et al. 2012). Its tolerance of disturbances and early seral conditions also make it a good choice for revegetation of burned rangeland sites where fire frequencies are expected to increase (IRFMS Team 2016). It is recommended for revegetation of all major land resource areas in Nevada (Eldredge et al. 2013) and at other sites within its range having medium to coarse-textured soils where the annual precipitation range is 9 to 15 in (230-380 mm) (Ogle et al. 2019). The species is well suited for seeding or transplanting in parks, along roadways, and other low maintenance landscapes.
The presence of Douglas’ dustymaiden in early post-fire communities (Wrobleski 1999; Ott et al. 2003) also make it desirable for revegetation of rangelands that have experienced increased fire frequencies in recent decades and require revegetation and management to improve resiliency (IRFMS Team 2016). Reliable early flowering suggests Douglas’ dustymaiden may be useful in post-fire seed mixes to provide food for bees in the first post-fire year (Cane 2012).
Douglas’ dustymaiden has colonized abandoned and reclaimed mine sites and other disturbances. It naturally colonized the ridgetops of a recontoured surface mine site in sagebrush steppe-salt desert shrub communities near Kemmerer, Wyoming. The last mining at this location occurred in the late 1970s, and rehabilitation began in 1982. Douglas’ dustymaiden was not seeded, but occurred on the site by 1985 (Hatton and West 1987). Douglas’ dustymaiden was one of the most common volunteer species on silver mine dumps near Park City, Utah, that were monitored about 45 years after surface mining operations ended (Alvarez et al. 1974). Similarly, it was one of the most successful volunteers on sulfide-bearing waste sites created during copper pit mining operations in Bingham Canyon, Utah (Borden and Black 2005). In Yellowstone National Park, Douglas’ dustymaiden is hand collected for use in seeding and container stock revegetation of sites disturbed during road construction (Majerus 1991).
Seeds of Douglas’ dusty maiden are not separated from the achene for use in restoration. Consequently, the term ‘seed’ as used by collectors, growers, and users refers to the fruits and that convention is applied in the following sections.
Developing A Seed Supply
For restoration to be successful, the right seed needs to be planted in the right place at the right time. Coordinated planning and cooperation is required among partners to first select appropriate species and seed sources and then properly collect, grow, certify, clean, store, and distribute seed for restoration (PCA 2015).
Developing a seed supply begins with seed collection from native stands. Collection sites are determined by current or projected revegetation requirements and goals. Production of nursery stock requires less seed than large-scale seeding operations, which may require establishment of agricultural seed production fields. Regardless of the size and complexity of any revegetation effort, seed certification is essential for tracking seed origin from collection through use (UCIA 2015).
Seed Sourcing
Because empirical seed zones are not currently available for Douglas’ dustymaiden, generalized provisional seed zones developed by Bower et al. (2014), may be used to select and deploy seed sources. These provisional seed zones identify areas of climatic similarity with comparable winter minimum temperature and aridity (annual heat:moisture index). In Figure 4, Omernik Level III Ecoregions (Omernik 1987) overlay the provisional seeds zones to identify climatically similar but ecologically different areas. For site-specific disturbance regimes and restoration objectives, seed collection locations within a seed zone and ecoregion may be further limited by elevation, soil type, or other factors.
The Western Wildland Environmental Threat Assessment Center’s (USFS WWETAC 2024) Threat and Resource Mapping (TRM) Seed Zone application provides links to interactive mapping features useful for seed collection and deployment planning. The Climate Smart Restoration Tool (Richardson et al. 2020) can also guide restoration planning, seed collection, and seed deployment, particularly when addressing climate change considerations.
Occurrence Map
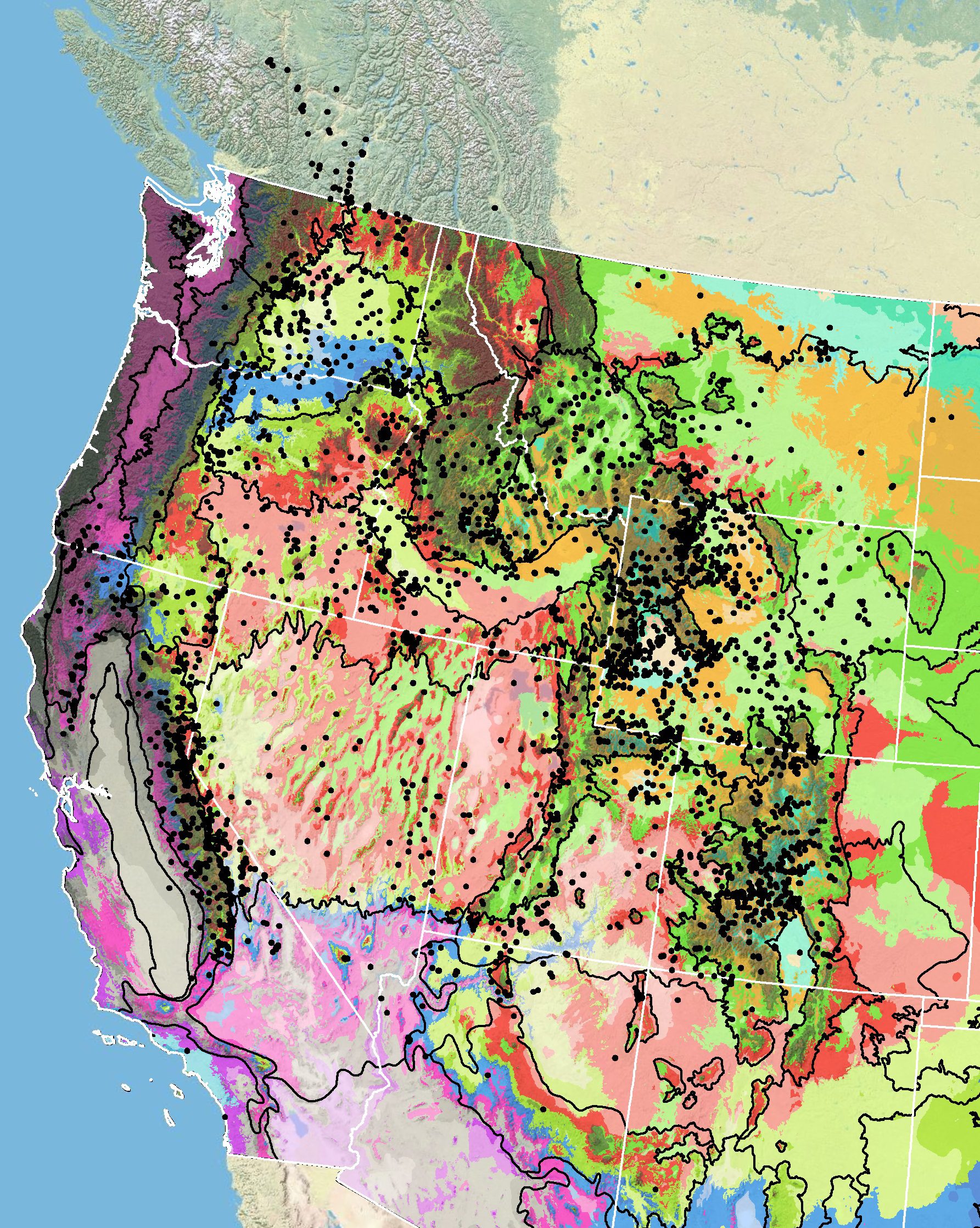
Figure 4. Distribution of Douglas’ dustymaiden (black circles) based on geo-referenced herbarium specimens and observational data from 1881-2016 (CPNWH 2017; SEINet 2017; USGS 2017). Generalized provisional seed zones (colored regions) (Bower et al. 2014) are overlain by Omernik Level III Ecoregions (black outlines) (Omernik 1987; USDI EPA 2018). Interactive maps, legends, and a mobile app are available (USFS WWETAC 2024). Map prepared by M. Fisk, USGS.
Releases
As of 2024, there were no Douglas’ dusty maiden germplasm releases.
Wildland Seed Collection
Wildland seed is collected by hand when the dandelion-like seed heads are white and spherical and the achenes are hard (Tilley 2010; B. Youtie, EOSS, personal communication, July 2017). Because Douglas’ dustymaiden seed is commercially available for revegetation and horticultural uses, this species may be found outside its native range (Morefield 2006), and these seeded sites should be avoided when making wildland collections.
Wildland Seed Certification
Verification of species and tracking of geographic source is necessary whether wildland seed is collected for immediate project use or as stock seed for cultivated increase. This official Source Identification process can be accomplished by following procedures established by the Association of Official Seed Certifying Agencies (AOSCA) Pre-Variety Germplasm Program (UCIA 2015; Young et al. 2020). Wildland seed collectors should become acquainted with state certification agency procedures, regulations, and deadlines in the states where they collect.
If wildland-collected seed is to be sold for direct use in ecological restoration projects, collectors must apply for Source-Identified certification prior to making collections. Pre-collection applications, site inspections, and species and seed amount verification are handled by the AOSCA member state agency where seed collections will be made (see listings at AOSCA.org).
If wildland seed collected by a grower or private collector is to be used as stock seed for planting cultivated seed fields or for nursery propagation (See Agricultural Seed Field Certification section), detailed information regarding collection site and collecting procedures must be provided when applying for certification. Photos and herbarium specimens may be required. Germplasm accessions acquired within established protocols of recognized public agencies, however, are normally eligible to enter the certification process as stock seed without routine certification agency site inspections. For contract grow–outs, however, this collection site information must be provided to the grower to enable certification.
Collection Timing
Timing of flowering and seed set is influenced by elevation, aspect, and seasonal weather patterns. Seed maturation generally occurs 4 to 5 weeks after flowering (Parkinson and DeBolt 2005) and varies widely among plants within a population and within individual plants (Fig. 5) (B. Youtie, Eastern Oregon Stewardship Services [EOSS], personal communication, July 2017). Northern populations generally flower later than those from more southern latitudes. In the same year (2010), nearly half of plants observed in central and northern Utah were blooming on June 14 but only 2% of plants in southern and central Idaho were flowering on the same date (Tilley 2011). Similarly, at the USDA NRCS Aberdeen Plant Materials Center (IDPMC) in southeastern Idaho, flowering in a common garden began in early summer and lasted for several weeks (Tilley 2010). Plants from more northern collection sites flowered later than plants from more southern collection sites.
Estimates of seed maturation and fill to determine adequacy of sites for collection and collection timing can be made using a cut test or X-ray test. The ‘pop test’ described by Tilley et al. (2011) may be a useful predictor of seed fill (See Seed Testing section).

Figure 5. Uneven seed ripening is typical for Douglas’ dustymaiden. Photo: M. Fisk, USGS.
The BLM’s Seeds of Success program made 69 collections from seven western states in 15 years (2002-2023) (USDI BLM SOS 2017, 2024). Collections were made in Idaho (19), Oregon (16), Nevada (11), California (9), Utah (9), Washington (2), and Wyoming (2). Fourteen harvests were made in June, 43 in July, nine in August, and one each in September and October. The earliest collection was made on June 9, 2009 in Owyhee County, Idaho, at 3,432 ft (1,046 m) elevation. The latest collection occurred on October 7, 2010 in Jackson County, Oregon, at 6,142 ft (1,872 m). The years with the greatest number of collections (14 each) were 2010 and 2018. In 2010, the earliest harvest was on June 19 at 5,066 ft (1,544 m) elevation in Millard County, Utah, and the latest was on October 7 at 6,142 ft (1,872 m) elevation in Jackson County, Oregon. In 2018, the earliest collection was on July 1 at 5,120 ft (1,561 m) in Washoe County Nevada, and the latest was on August 2 at 5,900 ft (1,798 m) in Washoe County, Nevada. At several sites, there were subsequent collections made after first although only the first dates of collection are recorded in the timing details above. Subsequent collections were made between a few days up to 6 weeks of the first (USDI BLM SOS 2017, 2024).
Collection Methods
Harvest method differs with the stage of ripening as this varies widely within individual plants and populations (B. Youtie, EOSS, personal communication, July 2017). When collecting seed at the early to mid-ripening stage when not all seed heads have matured, seeds can be hand plucked from the mature flower heads. This process is slow, it generally precludes collection of large quantities of seed, and it will include only early ripening seed. Later in the ripening season when most seeds have matured and have begun to disperse, seeds can be stripped, shaken, or knocked into a container (Tilley 2010; B. Youtie, EOSS, personal communication, July 2017). If seed can only be collected on one date, collection at the time of mid-ripening would enable collection of more early, mid- and late-ripening seed, thus providing greater genetic diversity and yield. Seeds can also be harvested by clipping entire seed heads (Link 1993). However, considerably less inert material is collected when using the plucking and stripping/shaking methods, thus simplifying the cleaning process (St. George 2003 cited in Camp and Sanderson 2007; Tilley 2010). Pappus removal may still be required, depending upon intended seed use. Gloves should be worn when collecting as the seed heads are sticky (A. Malcomb, USFS RMRS, personal communication, June 2017).
Several collection guidelines and methods should be followed to maximize the genetic diversity of wildland collections: 1) collect seed from a minimum of 50 randomly selected plants; 2) collect from widely separated individuals throughout a population without favoring the most robust or avoiding small statured plants; and 3) collect from all microsites including habitat edges (Basey et al. 2015). General collecting recommendations and guidelines are provided in online manuals (e.g., ENSCONET 2009; USDI BLM SOS 2023).
It is critical that wildland seed collection does not impact the sustainability of native plant populations. Collectors should take no more than 20% of the viable seed available at the time of harvest (USDI BLM SOS 2023). Additionally, care must be taken to avoid the inadvertent collection of weedy species, particularly those that produce seeds similar in shape and size to those of Douglas’ dustymaiden.
Collection Rates
In Yellowstone National Park, rates for hand-collected seed averaged 0.33 lbs (148 g)/hour (Majerus 1991). Neither the collection method nor the experience level of the collectors was reported.
Post-Collection Management
When collections are made in fall, Douglas’ dustymaiden seed heads and stems can have a high moisture content, and in general, the more plant material in the collection, the more ventilation and drying a seed lot will likely need (Gold n.d.; Parkinson and DeBolt 2005; Hay and Probert 2011). Harvested seed should be spread on racks in a protected area and thoroughly air dried in the field or following transport to the cleaning facility. Seed should dry in open collection sacks within 2 to 4 weeks (St. George 2003 cited in Camp and Sanderson 2007; Tilley 2010). Collected material can be transported in clean breathable bags or boxes but must be protected from overheating. Insect infestations should be controlled by freezing collections for 48 hours (Parkinson and DeBolt 2005) or use of appropriate chemicals.
Seed Cleaning
Seeds collected by plucking them from seed heads or by knocking or shaking the plants will require less cleaning than seeds collected by clipping the seed heads (Tilley 2010). Selection of cleaning procedures also depends upon whether the pappus must be removed from the seed. Seeds with the pappus attached can be seeded through grain and no-till drills if they are mixed with a diluent such as rice hulls (Tilley 2010).
The Bridger Montana Plant Materials Center (MTPMC) recommended that seed lots be threshed with a hammermill, then cleaned with an office or larger model air screen cleaner. They noted that cleaning to a flowable condition was difficult and the seed lot remained bulky after cleaning (Link 1993).
The IDPMC found that seed can be removed from stems and seed heads using a hammermill with a 0.2-in (0.6 cm) screen. The pappus can be removed using a debearder or brush machine. Seed is then fine cleaned with a multi-deck air screen cleaner followed by an indent cleaner (Tilley 2013).
The USFS Bend Seed Extractory, provided the following seed cleaning instructions based on one small seed lot (Barner 2009):
1. Process seed with a Westrup Model LA-H laboratory brush machine using a #40 mantel and speed of 3 to remove seed from the seed heads.
2. Finish seeds by air-screening to remove nonviable seed and inert material using an office Clipper with top screen #12 triangle (2nd run, 3 × 5/16 cross slot) and bottom screen blank at medium speed and low to medium air.
Seed Storage
Cool dry storage is recommended for clean Douglas’ dustymaiden seed. Dry seed can be stored at 33 to 38 °F (1-4 °C) (Barner 2009) and retain good viability for up to 5 years (Link 1993).
Seed Testing
There is no Association of Official Seed Analysts (AOSA) rule for testing germination or protocol for examining viability of Douglas’ dustymaiden seed (AOSA 2010, 2023). Purity is evaluated using standard AOSA procedures.
Quick estimates of seed fill can be made using the ‘pop test’ described by Tilley et al. (2011), which uses a hot plate to heat seeds until the moisture contained in the seed is converted to gas and breaks the seed coat, producing a pop.
Germination Biology
Douglas’ dustymaiden seed is considered deeply dormant, and long periods of cold stratification are typically required to break dormancy (Barga et al. 2017; Kildisheva et al. 2019). Warm temperatures following stratification encourage germination. In laboratory tests, germination was greater after 60 or 90 days of cool moist stratification at 36 °F (2 °C) (27-30.5%) compared to 0 or 30 days (0.5-14%) (Tilley 2013). In other laboratory tests, cool afterripening resulted in earlier and greater total germination when warm temperatures followed cool moist seed stratification. Douglas’ dustymaiden seed for these tests came from three populations collected near Reno and Carson City, Nevada, and exhibited population differences related to post-stratification incubation temperatures (Leger and Barga 2015). For nursery stock production, the USFS Lucky Peak Nursery (LPN) used a 24-hour cold water soak followed by a 30-day cold moist stratification to release dormancy (P. Winn, USFS LPN, personal communication, July 2017).
Barga et al. (2017) found that longer periods of cold stratification enhanced germination of Douglas’ dustymaiden. Cool afterripening also resulted in faster and higher total germination. Germination was often quicker when warm temperatures followed cold stratification. This study evaluated seed collected at various times from three populations in northern Nevada in an attempt to capture the reproductive window. One population germinated much faster than the others, suggesting that germination requirements may vary by seed lot. Seed was stored in the dark at 70 °F (21 °C) until tested. Viability of the wild-collected seed was 90 to 97.5%. Germination treatments included 4 weeks of cool 70 °F (21 °C) or hot afterripening 104 °F (40 °C), 0 to 6 weeks of cold moist stratification 36 °F (2 °C), and incubation at 59 °F (15 °C) (Barga et al. 2017).
In a study by Kildisheva et al. (2019), maximum germination of Douglas’ dustymaiden was just 4% without pretreatment or exposure to chemical stimulants (karrikinolide [KAR1] and gibberellic acid [GA3]) at incubation temperatures of 59/41, 68/50, or 77/59 °F (15/5, 20/10, 25/15 °C). In this study, neither afterripening or dry cold storage -0.4 °F (-18 °C) reduced seed dormancy of seed that was collected at 5,728 ft (1,746 m) in Washoe County, Nevada. Germination improvements were evident after 2 months of cold stratification at 37 °F (3 °C) (P ≤ 0.001). Three months of cold stratification generally promoted maximum germination of Douglas’ dustymaiden at the warmest incubation temperatures (77/59 °F [25/15 °C]). Moderate germination (32-39%) of seeds maintained at 37 to 41 °F (3-5 °C) after just one month suggests that the requirements to break seed dormancy may be met before the end of winter in the field and some germination may occur before the danger of frost has passed. Germination of Douglas’ dustymaiden seed incubated at continuous 37 to 41 °F (3-5 °C) increased with time, and after 6 months, most seeds had germinated with no additional benefit to germination by exposing seeds to warmer temperatures (Kildisheva et al. 2019). Although germination at low temperatures may benefit seedling competitive ability (Leger and Barga 2015), high frost risk during this period can also cause mortality and may, at least in part, contribute to restoration failures in the Great Basin.
Wildland Seed Yield And Quality
Post-cleaning seed yield and quality of seed lots collected in the Intermountain region are provided in Table 1 (USFS BSE 2017, 2023). The results indicate that Douglas’ dustymaiden seed can generally be cleaned to high levels of purity and seed fill and that viability of fresh seed is generally high. Barner (2009) also reported high purity (97%), seed fill (92%), and viability (79%) for cleaned seed. Shock et al. (2014a) reported viability of harvested seed from cultivated research plots was typically about 70%.
Douglas’ dustymaiden seeds are small, averaging more than 350,000 seeds/lb (770,000/kg) (Table 1). Other sources report similar values, which ranged from 300,000 to 450,000 seeds/lb (660,000–990,000 seeds/kg) (Majerus 1991; Lambert 2005; Parkinson and DeBolt 2005; Tilley 2013; Shock et al. 2014a; SER, INSR, RBGK 2024).
Table 1. Seed yield and quality of Douglas’ dustymaiden seed lots collected in the Intermountain region, cleaned and evaluated by the Bend Seed Extractory. Viability was tested by the Oregon State Seed Laboratory or the USFS National Seed Laboratory (USFS BSE 2017, 2023).
|
Seed lot characteristic |
Mean |
Range |
Samples (no.) |
|
Bulk weight (lbs) |
0.81 |
0.04-9.0 |
101 |
|
Clean weight (lbs) |
0.13 |
0.002-1.5 |
101 |
|
Clean–out ratio |
0.19 |
0.015-0.7 |
101 |
|
Purity (%) |
96 |
78-98 |
100 |
|
Fill (%)¹ |
93 |
70-99 |
101 |
|
Viability (%)² |
92 |
79-99 |
67 |
|
Seeds/lb |
374,723 |
210,780–620,000 |
101 |
|
Pure live seeds/lb |
326,999 |
208,802-565,440 |
67 |
¹100 seed X–ray test
²Tetrazolium chloride test
Marketing Standards
Acceptable seed purity, viability, and germination specifications vary with revegetation plans. Purity needs are highest for precision seeding equipment like that used in nurseries, while some rangeland seeding equipment handles less clean seed quite well.
Agricultural Seed Production
In cultivated stands plants flower in their first year (Fig. 6) (Cane 2012; Shock et al. 2014c). At Oregon State University’s Malheur Experiment Station (OSU MES), flowering began on May 23 and peaked on June 30. Seed was harvested on July 2 and again on July 22 in one harvest year (Shock et al. 2014c).
Seed production beyond the first year is variable. At the IDPMC, seed yield ratings for 15 accessions of first-year plants ranged from 3 to 5 on a scale of 1 to 9, where 1 indicated highest seed yields. While all plants regrew the following year, just 4 of 15 accessions produced flowers on more than 30% of the plants by mid-June (Tilley 2011). At OSU MES, Douglas’ dustymaiden seed production was five to eight times greater for first-year than for second-year plants (Shock et al. 2017b).
Agricultural Seed Certification
In order to minimize genetic changes in specific accessions of native species when increased in cultivated fields, it is essential to track the geographic source and prevent inadvertent hybridization or selection pressure. This is accomplished by following seed certification protocols for Pre-Variety Germplasm (PVG) as established by the Association of Official Seed Certification Agencies (AOSCA). AOSCA members in the U.S., Canada, and other countries administer PVG requirements and standards that track the source and generation of planting stock. Field and cleaning facility inspections are conducted to monitor stand establishment, proper isolation distances, control of prohibited weeds, seed harvesting, cleaning, sampling, testing, and labeling for commercial sales (UCIA 2015; Young et al. 2020).
Seed growers apply for certification of their production fields prior to planting and plant only certified stock seed of an allowed generation (usually less than four). The systematic and sequential tracking through the certification process requires preplanning, knowing state regulations and deadlines, and is most smoothly navigated by working closely with state certification agency personnel. See the Wildland Seed Certification section for more information on stock seed sourcing.

Figure 6. Douglas’ dustymaiden seed production plot at the USFS Lucky Peak Nursey. Photo: J. Cane, USDA ARS.
Site Preparation
Douglas’ dustymaiden should be planted in a weed-free seedbed.
Seed Pretreatments
In experimental establishment studies conducted at OSU MES, Douglas’ dustymaiden seed was pre-treated with a liquid mix of sulphur fungicides prior to planting (Shock et al. 2014a). Treated seeds typically produced better first-year stands than untreated seeds (Shock et al. 2017a).
Weed Management
In studies of cultivated stands at OSU MES, none of the preemergence herbicides tested significantly reduced emergence of Douglas’ dustymaiden when compared to untreated controls (Shock et al. 2014b). However, this was when the effects of all herbicide treatments were averaged. Notably, percent emergence was 27.5% for untreated stands, 47% for stands treated with an S-ethyl dipropylthiocarbamate, and 6.8% for stands treated with dimethenamid-P. Across all post-emergence herbicides, stand loss was 4.8% in untreated stands and 5.8% in treated stands (Shock et al. 2014b). Weedy grasses were controlled with a selective herbicide at IDPMC (Tilley 2010), but neither the herbicide nor its active ingredient were named.
Seeding
The IDPMC developed general guidelines for seeding Douglas’ dustymaiden (Tilley 2010). Late fall seeding allowed for natural stratification. Seeds were planted in rows 9 to 18 in (23-45 cm) apart into slightly roughened soil. Soil was then lightly packed around the deposited seeds. A target rate of 12 to 25 seeds/hole was recommended. A custom made “penstemon popper” seeder was used to manually plant Douglas’ dustymaiden. It consists of a 3-in (7.6 cm) diameter tube with a spur and a foot at the bottom. The spur roughened the soil before seeds were dropped down the tube. The foot then lightly compacted the soil around the seed (Tilley 2010).
At OSU MES, stands were established by seeding Douglas’ dustymaiden in 30-in (76 cm) rows using a custom small-plot grain drill with disk openers. Seed was planted on the soil surface at a rate of 20 to 30 seeds/ft (66-98/m) of row. Sawdust was applied in a narrow band over the seed at a rate of 0.26 oz/ft (24 g/m) of row. However, stand establishment was not successful after every seeding and to develop a good stand, hand planting of Douglas’ dustymaiden in the thin sections was necessary (Shock et al. 2017b).
Establishment And Growth
Fall, winter, or early spring field seeding of Douglas’ dustymaiden has resulted in successful stand establishment. In a breeding biology study, stands established from seeds planted under snow in March 2009 (Cane et al. 2012). Cultivated Douglas’ dustymaiden stands were established from fall seeding at the IDPMC (Tilley 2011). Establishment ranged from 66 to 98% for 15 accessions of Douglas’ dustymaiden seed collected across the Intermountain West (UT, ID, and OR). The accession with the poorest establishment produced the tallest plants with the most flowers and had a high seed yield rating. Plots were established in Declo silt loam soils with a pH of 8.4, and Douglas’ dustymaiden was seeded into weed barrier fabric. There were 12 to 25 seeds/hole, and holes were 9 in (23 cm) apart (Tilley 2011).
Over 3 years of testing various direct seeding systems at OSU MES, row cover consistently improved Douglas’ dustymaiden stand production. When emergence trial results were averaged over the 3 years, the application of sand to keep the seed in place beneath the row cover yielded the best stands (Shock et al. 2017a).
Irrigation
In irrigation studies conducted at OSU MES, Douglas’ dustymaiden seed yield varied greatly over a 2-year period, but was not related to irrigation rates. Irrigation was applied at about 2-week intervals from bud formation through flowering (Table 2; Shock et al. 2017b).
Table 2. Douglas’ dustymaiden seed yield (lb/ac) with and without supplemental irrigation in seed production fields at Oregon State University’s Malheur Experiment Station in Ontario, OR (Shock et al. 2017b).
|
Year |
Irrigation Rate |
||
|
0 in |
4 in |
8 in |
|
|
Yield (lbs/ac) |
|||
|
2015 |
132.1 |
137.6 |
183.3 |
|
2016 |
29.1 |
16.0 |
27.2 |
|
Average |
80.6 |
76.8 |
105.2 |
Pollinator Management
The most practical, manageable bees to use to pollinate farmed crops of Douglas’ dustymaiden are the cavity-nesting Asteraceae specialist (Osmia californica) and the European honey bee (Apis mellifera) (Cane et al. 2012), which could be transported to field locations in portable ground nests or hives. However, introducing new bee populations may not be feasible and encouraging and sustaining native bee populations where present can benefit production of many native plant crops (Cane 2008).
Seed Harvesting
Combines, flailvacs, or vacuum-type harvesters were used to collect Douglas’ dustymaiden seed at the IDPMC. Jet combines (Jet Harvester, Aberdeen, ID) with fans running at 6,000 rpm ensured only ripe seed was harvested and allowed for multiple harvests throughout the season. Jet combine collections included only limited amounts of chaff and other inert material and made post-harvest cleaning easier (Bair and Tilley 2010; Tilley 2010). At OSU MES, Douglas’ dustymaiden seed was collected from small research plots by hand or using a leaf blower in vacuum mode. Because of the long flowering duration, beginning in early May and ending mid-July, seed was collected weekly from mid-June to mid-July (Shock et al. 2017b).
Researchers at the IDPMC developed a unique non-destructive seed collection method for species producing indeterminate lightweight seed, which could be used for Douglas’ dustymaiden (Simonson and Tilley 2016). A system of loose chains was attached to the hood of a flail-vacuum harvester. The chains agitated the plants and dislodged ripe seed from the flower heads when passed through seed production rows. This harvest method resulted in limited plant damage, allowed for repeat harvests, and collected only a minimal amount of vegetative material (Simonson and Tilley 2016).
Seed Yields And Stand Life
In cultivated stands at OSU MES, Douglas’ dustymaiden flowered in its first year and seed production was 5 to 8 times greater for first year plants than for second year plants (Shock et al. 2017b). At the IDPMC, seed yield for first-year Douglas’ dustymaiden plants were rated 3, 4, or 5 for 15 accessions. Ratings were based on a visual 1 to 9 rating, where 1 indicated highest yields (Tilley 2011).
Nursery Practice
Containerized stock of Douglas’ dustymaiden material has been produced for domestic horticulture and for restoration (Fig. 7) (Majerus 1991; Link 1993; Tilley 2011; Shock et al. 2014a).
Container plants were produced in Boise, Idaho, by placing sown containers outside in December (DeBolt and Barrash 2013). Emergence began after 2 months and was complete after 4 months. Seedlings were transferred to 5.5-in (14 cm) containers when secondary leaves developed. Seedlings were grown outside for 6 months, and when observed, flower stalks were removed to promote vegetative growth. Seedlings were outplanted in October (DeBolt and Barrash 2013).

Figure 7. Container-grown Douglas’ dustymaiden seedlings. Photo: P. Winn, USFS LPN.
Wildland Seeding And Planting
Based on experience gained when growing seed crops of Douglas’ dustymaiden, the IDPMC recommended a seeding rate of 3 lbs of pure live seed (PLS)/ac (3.4 kg PLS/ha) in fall at a seeding depth of 0 to 0.1 in (0-0.3 cm) (Ogle et al. 2011, 2012). The 3 lbs/ac (3.4 kg/ha) seeding rate is recommended for establishment of a pure stand, but Douglas’ dustymaiden use in rangeland seedings is most likely as a component of seeding mixtures, making up less than 10% of the mix. Sites with medium- to coarse-textured soils with a pH of 4.2 to 8.0 that receive at least 7 in (180 mm) of annual precipitation are considered best for Douglas’ dustymaiden establishment and growth (Ogle et al. 2011, 2012).
At Curlew National Grassland in Oneida County, Idaho, 58 species of various native and nonnative grasses, forbs and shrubs were seeded at a site that prior to burning was a mountain big sagebrush/bluebunch wheatgrass community (Tilley et al. 2022). The site burned in 2006, was plowed and packed in fall 2009, and treated with herbicide in 18 June and 29 July in 2010. A mix containing Douglas’ dustymaiden was drill seeded using a modified Tye grain drill in rows spaced 10 in (25 cm) apart on November 17, 2010, at a rate of 42 to 46 PLS/ft² (450-500/m²). Plots were mowed to height of about 4 in (10 cm) on September 29, 2011, after which there were no other weed control, fertilizer, or water treatments. Density of Douglas’ dustymaiden was a little more than 0.3 plants/ft² (4 plants/m²) on July 11, 2011, almost 0.2 plants/ft² (2 plants/m²) in 2012, and decreased from there. There were no Douglas’ dustymaiden plants in 2014 or in 2020 (10 yrs after seeding). The source of Douglas’ dustymaiden seed used in this study was not reported (Tilley et al. 2022).
Acknowledgements
Funding for Western Forbs: Biology, Ecology, and Use in Restoration was provided by the USDI BLM Great Basin Native Plant Materials Ecoregional Program through the Great Basin Fire Science Exchange. Great thanks to the chapter reviewers: Jim Cane, USDA ARS, Clint Shock, OSU MES, and Jeremy Pinto, USFS.
This research was supported in part by the USDA Forest Service, Rocky Mountain Research Station. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA or U.S. Government determination or policy.
Literature Cited
Agneray, A.C.; Parchman, T.L.; Leger, E.A. 2022. Phenotypes and environment predict seedling survival for seven co-occurring Great Basin plant taxa growing with invasive grass. Ecology and Evolution. 12(5): e8870.
Alvarez, H.; Ludwig, J.; Harper, K.T. 1974. Factors influencing plant colonization of mine dumps at Park City, Utah. The American Midland Naturalist. 92(1): 1-11.
Andersen, B.A.; Holmgren, A.H. 1996. Mountain plants of northeastern Utah, revised. HG 506. Logan, UT: Utah State University Extension. 132 p.
Association of Official Seed Analysts [AOSA]. 2010. AOSA/SCST Tetrazolium testing handbook. Contribution No. 29. Lincoln, NE: Association of Official Seed Analysts.
Association of Official Seed Analysts [AOSA]. 2023. AOSA rules for testing seeds. Vol. 1. Principles and procedures. Washington, DC: Association of Official Seed Analysts.
Bair, C.; Tilley, D. 2010. The jet harvester: A shop-built tool for harvesting forb and shrub seed. Technical Note 55. Boise ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 6 p.
Barga, S.; Dilts, T.E.; Leger, E.A. 2017. Climate variability affects the germination strategies exhibited by arid land plants. Oecologia. 185: 437-452.
Barga, S.; Leger, E.A. 2018. Shrub cover and fire history predict seed bank composition in Great Basin shrublands. Journal of Arid Environments. 154: 40-50.
Barga, S.C.; Dilts, T.E.; Leger, E.A. 2018. Contrasting climate niches among co-occurring subdominant forbs of the sagebrush steppe. Diversity and Distributions. 24(9/10): 1291-1307.
Barner, J. 2009. Propagation protocol for production of propagules (seeds, cuttings, poles, etc.) Chaenactis douglasii (Hook.) Hook. & Arn. seeds. Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://npn.rngr.net/propagation/protocols [Accessed 2017 April 23].
Basey, A.C.; Fant, J.B.; Kramer, A.T. 2015. Producing native plant materials for restoration: 10 rules to collect and maintain genetic diversity. Native Plants Journal. 16(1): 37-53.
Borden, R.; Black, R. 2005. Volunteer revegetation of waste rock surfaces at the Bingham Canyon Mine, Utah. Journal of Environmental Quality. 34: 2234-2242.
Bower, A.D.; St. Clair, J.B.; Erickson, V. 2014. Generalized provisional seed zones for native plants. Ecological Applications. 24(5): 913-919.
Camp, P.; Sanderson, J. 2007. Seed collection, propagation and reintroduction of native wildflowers in the Columbia Basin. Wenatchee, WA: U.S. Department of the Interior, Bureau of Land Management, Wenatchee Field Office. 32 p.
Cane, J.H. 2008. Pollinating bees crucial to farming wildflower seed for U.S. habitat restoration. In: James, R.; Pitts-Singer, T., eds. Bees in agricultural ecosystems. Oxford, UK: Oxford University Press: 48-64.
Cane, J.H. 2012. Pollination and breeding biology studies. In: Shaw, N.; Pellant, M., eds. Great Basin Native Plant Project: 2011 Progress Report. Boise, ID: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 173-176.
Cane, J.H.; Love, B.; Swoboda, K. 2012. Breeding biology and bee guild of Douglas’ dustymaiden, Chaenactis douglasii (Asteraceae, Helenieae). Western North American Naturalist. 72(4): 563-568.
Chamberlin, R.V. 1911. The ethno-botany of the Gosiute Indians. American Anthropological Association. 63(1): 329-405.
Chambers, K.L. 2020. Dicots: Asteraceae. In: Meyers, S.C.; Jaster, T; Mitchell, K.E.; Harvey, T.; Hardison, L.K., eds. Flora of Oregon. Volume 2: Dicots A-F. Fort Worth, TX: Botanical Research Institute of Texas (BRIT): 152-370.
Consortium of Pacific Northwest Herbaria [CPNWH]. 2017. Seattle, WA: University of Washington Herbarium, Burke Museum of Natural History and Culture. http://www.pnwherbaria.org/index.php
Craighead, J.J.; Craighead, F.C. Jr.; Davis, R.J. 1963. A field guide to Rocky Mountain wildflowers from northern Arizona and New Mexico to British Columbia. Boston, MA: Houghton Mifflin Company. 277 p.
Cronquist, A. 1994. Volume five: Asterales. In: Cronquist, A.; Holmgren, A.H.; Holmgren, N.H.; Reveal, J.L.; Holmgren, P.K., eds. Intermountain flora: Vascular plants of the Intermountain West, U.S.A. Bronx, NY: The New York Botanic Garden. 496 p.
Davis, J.; Harper, K. 1990. Weedy annuals and establishment of seeded species on a chained juniper-pinyon woodland in central Utah. In: McArthur, E.D.; Romney, E.; Smith, S.; Tueller, P., comps. Proceedings-Symposium on cheatgrass invasion, shrub die-off, and other aspects of shrub biology and management; 1989 April 5-7; Las Vegas, NV. Gen. Tech. Rep. INT-GTR-276. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Research Station: 72-79.
Day, T.; Wright, R.G. 1985. The vegetation types of Craters of the Moon National Monument. Bull. 38. Moscow, ID: University of Idaho, College of Forestry, Wildlife, and Range Sciences, Forest, Wildlife and Range Experiment Station. 6 p.
DeBolt, A.M.; Barrash, K. 2013. Propagation protocol for production of container (plug) Chaenactis douglasii (Hook.) Hook. & Arn. plants 2.875-inch x 5.5-inch plant band (container). Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://NativePlantNetwork.org [Accessed 2017 April 23].
Eldredge, E.; Novak-Echenique, P.; Heater, T.; Mulder, A.; Jasmine, J. 2013. Plants for pollinator habitat in Nevada. Tech. Note NV 57. Reno, NV: U.S. Department of Agriculture, Natural Resources Conservation Service. 65 p.
Ellsworth, L.M.; Kauffman, J.B. 2013. Seedbank responses to spring and fall prescribed fire in mountain big sagebrush ecosystems of differing ecological condition at Lava Beds National Monument, California. Journal of Arid Environments. 96: 1-8.
European Native Seed Conservation Network [ENSCONET]. 2009. ENSCONET seed collecting manual for wild species. Edition 1: 32 p.
Friday, C.; Scasta, J.D. 2020. Eastern Shoshone and Northern Arapaho traditional ecological knowledge (TEK) and ethnobotany for Wind River Reservation rangelands. Ethnobiology Letters. 11(1): 14-24.
Gold, K. n.d. Post-harvest handling of seed collections. Technical Information Sheet 04. UK: Royal Botanic Gardens Kew and Millennium Seed Bank Partnership. 4 p.
Hatton, T.; West, N. 1987. Early seral trends in plant community diversity on a recontoured surface mine. Vegetatio. 73(1): 21-29.
Hay F.R.; Probert, R.J. 2011. Chapter 20: Collecting and handling seeds in the field. In: Guarino, L.; Ramanatha, V.; Goldberg, E. Collecting plant genetic diversity: Technical Guidelines-2011 update. Rome, Italy: Bioversity International. 33 p.
Hickman, J.C., ed. 1993. The Jepson manual: Higher plants of California. Berkeley, CA: University of California Press. 1400 p.
Hitchcock, C.L.; Cronquist, A. 2018. Flora of the Pacific Northwest: An illustrated manual. Second Ed. Giblin, D.E.; Legler, B.S.; Zika, P.F.; Olmstead, R.G., eds. Seattle, WA: University of Washington Press. 882 p.
Huffman, D.W.; Stoddard, M.T.; Springer, J.D.; Crouse, J.E. 2017. Understory responses to tree thinning and seeding indicate stability of degraded pinyon-juniper woodlands. Rangeland Ecology and Management. 70: 484-492.
Integrated Rangeland Fire Management Strategy Actionable Science Plan Team [IRFMS Team]. 2016. The Integrated Rangeland Fire Management Strategy Actionable Science Plan. Washington, DC: U.S. Department of the Interior. 128 p.
Kildisheva, O.A.; Erickson, T.E.; Kramer, A.T.; Zeldin, J.; Merritt, D.J. 2019. Optimizing physiological dormancy break of understudied cold desert perennials to improve seed-based restoration. Journal of Arid Environments. 170: 104001.
Koniak, S. 1985. Succession in pinyon-juniper woodlands following wildfire in the Great Basin. Great Basin Naturalist. 45(3): 556-566.
Lady Bird Johnson Wildflower Center [LBJWC]. 2017. Chaenactis douglasii (Hook.) Hook. & Arn. Native Plant Database. Austin, TX: Lady Bird Johnson Wildflower Center. https://www.wildflower.org/plants-main [Accessed 2017 September 13].
Lambert, S. 2005. Guidebook to the seeds of native and non-native grasses, forbs and shrubs of the Great Basin. Boise, ID: U.S. Department of the Interior, Bureau of Land Management, Idaho State Office. 136 p.
Leger, E.; Barga, S. 2015. Species and population-level variation in germination strategies of cold desert forbs. In: Kilkenny, F; Halford, A.; Malcomb, A., eds. Great Basin Native Plant Project: 2014 Progress Report. Boise, ID: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 37-41.
Link, E., ed. 1993. Native plant propagation techniques for national parks interim guide: A cooperative program between the U.S. Department of Agriculture, Soil Conservation Service and U.S. Department of the Interior, National Park Service. East Lansing, MI: U.S Department of Agriculture, Natural Resources Conservation Service, Rose Lake Plant Materials Center.
Luna, T.; Mousseaux, M.R.; Dumroese, R.K. 2018. Common native forbs of the northern Great Basin important for greater sage-grouse. Gen. Tech. Rep. RMRS-GTR-387. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station; Portland, OR: U.S. Department of the Interior, Bureau of Land Management, Oregon-Washington Region. 76 p.
Mahar, J.M. 1953. Ethnobotany of the Oregon Paiutes of the Warm Springs Indian Reservation. Portland, OR: Reed College. Thesis. 143 p.
Majerus, M. 1991. Yellowstone National Park-Bridger Plant Materials Center native plant program. In: Rangeland Technology and Equipment Council, ed. 1991 Annual Report. 9222-2808-MTDC. Missoula, MT: U.S. Department of Agriculture, Forest Service, Missoula Technology and Development Center, Technology and Development Program: 17-22.
Mooring, J.S. 1980. A cytogeographic study of Chaenactis douglasii (Compositae, Helenieae). American Journal of Botany. 67: 1304-1319.
Mooring, J.S. 1992. Chromosome numbers and geographic distribution in Chaenactis douglasii (Compositae, Helenieae). Madrono. 39: 263-270.
Morefield, J.D. 2006. 378. Chaenactis. In: Flora of North America Editorial Committee, ed. Flora of North America North of Mexico. Volume 21 Magnoliophyta: Asteridae, part 8: Asteraceae, part 3 Asterales, part 3 (Aster order). New York, NY: Oxford University Press: 400-414.
Nickerson, G.S. 1966. Some data on Plains and Great Basin Indian uses of certain native plants. Tebiwa. 9(1): 45-51.
Ogle, D.; Tilley, D.; Cane, J.; St. John, L.; Fullen, K.; Stannard, M.; Pavek, P. 2011. Plants for pollinators in the Intermountain West. Plant Materials Tech. Note 2A. Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 40 p.
Ogle, D.; Tilley, D.; Cane, J.; St. John, L.; Fullen, K.; Stannard, M.; Pavek, P. 2019. Plants for pollinators in the Intermountain West. Plant Materials Tech. Note 2A. Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 40 p.
Ogle, D; St. John, L.; Stannard, M.; Holzworth, L. 2012. Conservation plant species for the Intermountain West. Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 57 p.
Omernik, J.M. 1987. Ecoregions of the conterminous United States. Map (scale 1:7,500,000). Annals of the Association of American Geographers. 77(1): 118-125.
Ott, J.; McArthur; E.D.; Roundy, B. 2003. Vegetation of chained and non-chained seedings after wildfire in Utah. Journal of Range Management. 56: 81-91.
Parkinson, H.; DeBolt, A. 2005. Propagation protocol for production of container (plug) Chaenactis douglasii (Hook.) Hook. & Arn. plants. Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://NativePlantNetwork.org [Accessed 2017 April 23].
Pavek, P.; Erhardt, B.; Heekin, T.; Old, R. 2012. Forb seedling identification guide for the Inland Northwest: Native, introduced, invasive, and noxious species. Pullman, WA: U.S. Department of Agriculture, Natural Resources Conservation Service, Pullman Plant Materials Center. 144 p.
Pérez, F.L. 2012. Biogeomorphological influence of slope processes and sedimentology on vascular talus vegetation in the southern Cascades, California. Geomorphology. 138(1): 29-48.
Perry, F. 1952. Ethno-botany of the Indians in the Interior of British Columbia. Museum and Art Notes. 2(2): 36-43.
Pitt, M.D.; Wikeem, B.M. 1990. Phenological patterns and adaptations in an Artemisia/Agropyron plant community. Journal of Range Management. 43(4): 350-358.
Plant Conservation Alliance [PCA]. 2015. National seed strategy for rehabilitation and restoration 2015-2020. Washington, DC: U.S. Department of the Interior, Bureau of Land Management. 52 p.
Plants for a Future [PFAF]. 2024. Available: https://pfaf.org/user/Plant.aspx
Poudel, A.; Satyal, P.; Swor, K.; Setzer, W.N. 2023. Essential oils of two Great Basin composites: Chaenactis douglasii and Dieteria canescens from southwestern Idaho. Journal of Essential Oil and Plant Composition. 1(3): 275-283.
Ray, V.F. 1933. The Sanpoil and Nespelem: Salishan peoples of northeastern Washington. Seattle, WA: University of Washington Press. 237 p.
Richardson, B.; Kilkenny, F.; St. Clair, B.; Stevenson-Molnar, N. 2020. Climate Smart Restoration Tool. https://climaterestorationtool.org/csrt/
Rink, G.R.; Hodgson, W.; Phillips, B.G. 2020. Checklist of the vascular flora of the Kaibab Plateau, Coconino County, Arizona. Western North American Naturalist. 12(1): 1-52.
Robinson, G.S.; Ackery, P.R.; Kitching, I.; Beccaloni, G.W.; Hernández, L.M. 2023. HOSTS: A Database of the World’s Lepidopteran Hostplants [Data set]. Natural History Museum. https://doi.org/10.5519/havt50xw
SEINet – Regional Networks of North American Herbaria Steering Committee [SEINet]. 2017. SEINet Regional Networks of North American Herbaria. https://symbiota.org/seinet
Shemluck, M. 1982. Medicinal and other uses of the Compositae by Indians in the United States and Canada. Journal of Ethnopharmacology. 5: 303-358.
Shock, C.C.; Feibert, E.B.G.; Rivera, A.; Sanders, L.D.; Kilkenny, F.; Shaw, N. 2017a. Direct surface seeding systems for the establishment of native plants in 2016. In: Shock, C.C., ed. Malheur Experiment Station Annual Report 2016. OSU AES Ext/CRS 157. Corvallis, OR: Oregon State University: 123-130.
Shock, C.C.; Feibert, E.B.G.; Rivera, A.; Saunders, L.D. 2017b. Irrigation requirements for seed production of several native wildflower species planted in the fall of 2012. In: Shock, C.C., ed. Malheur Experiment Station Annual Report 2016. OSU AES Ext/CrS157. Corvallis, OR: Oregon State University: 131-140.
Shock, C.C.; Feibert, E.B.G.; Saunders, L.D. 2014a. Direct surface seeding strategies for successful establishment of native wildflowers. In: Shock, C.C., ed. Malheur Experiment Station Annual Report 2013. OSU AES Ext/CrS149. Corvallis, OR: Oregon State University: 159-165.
Shock, C.C.; Feibert, E.B.G.; Saunders, L.D.; Shaw, N. 2014b. Tolerance of native wildflower seedlings to preemergence and postemergence herbicides. In: Shock, C.C., ed. Malheur Experiment Station Annual Report 2013. OSU AES Ext/CrS149. Corvallis, OR: Oregon State University: 98-206.
Shock, C.C.; Feibert, E.B.G.; Saunders, L.D.; Shaw, N. 2014c. Irrigation requirements of annual native wildflower species for seed production, fall 2012 planting. In: Shock, C.C., ed. Malheur Experiment Station Annual Report 2013. OSU AES Ext/CrS149. Corvallis, OR: Oregon State University: 193-197.
Simonson, D.B.; Tilley, D.J. 2016. A low-cost modification to a Flail-Vac Harvester for collecting lightweight, wind-dispersed seed. Native Plants Journal. 17(2): 103-108.
Society for Ecological Restoration; International Network for Seed Based Restoration; Royal Botanic Gardens [SER, INSR, RBGK]. 2023. Seed Information Database (SID). Available from: https://ser-sid.org/
Steedman, E.V. 1928. Ethnobotany of the Thompson Indians of British Columbia: Based on field notes by James A. Teit. Smithsonian Institution. Bureau of American Ethnology Annual Report V. 45. Washington, D.C.: U.S. Government Printing Office. 80 p.
Tilley, D. 2010. Propagation protocol for production of propagules (seeds, cuttings, poles, etc.) Chaenactis douglasii (Hook.) Hook. & Arn. seeds. Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://NativePlantNetwork.org [Accessed 2017 April 23].
Tilley, D. 2011. Douglas’ dustymaiden initial evaluation planting-Final study report. Aberdeen Plant Materials Center. Aberdeen, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 9 p.
Tilley, D. 2013. Propagation protocol for production of container (plug) Chaenactis douglasii (Hook.) Hook. & Arn. plants. Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://NativePlantNetwork.org [Accessed 2017 April 23].
Tilley, D.; Ogle, D.; Cornforth, B. 2011. The pop test: A quick aid to estimate seed quality. Native Plants Journal. 12(3): 227-232.
Tilley, D.; Ogle, D.; St. John, L. 2010. Plant guide: Douglas’ dusty maiden (Chaenactis douglasii [Hook.] Hook. & Arn.). Aberdeen, ID: U.S. Department of Agriculture, Natural Resources Conservation Service, Aberdeen Plant Materials Center. 4 p.
Train, P.; Henrichs, J.R.; Archer, W.A. 1941. Medicinal uses of plants by Indian tribes of Nevada: Part 1. Contributions toward a flora of Nevada. No. 33. Washington, DC: U.S. Department of Agriculture, Bureau of Plant Industry, Division of Plant Exploration and Introduction. 199 p.
Turner, N.J.; Bouchard, R.; Kennedy, D.I. 1980. Ethnobotany of the Okanagan-Colville Indians of British Columbia and Washington. Victoria, BC: British Columbia Provincial Museum. 179 p.
USDA Forest Service, Bend Seed Extractory [USDA FS BSE]. 2017. Nursery Management Information System Version 4.1.11. Local Source Report 34-Source Received. Bend, OR: U.S. Department of Agriculture, Forest Service, Bend Seed Extractory.
USDA Forest Service, Bend Seed Extractory [USDA FS BSE]. 2023. Nursery Management Information System Version 4.1.11. Local Source Report 34-Source Received. Bend, OR: U.S. Department of Agriculture, Forest Service Bend Seed Extractory.
USDA Forest Service, Western Wildland Environmental Threat Assessment Center [USFS WWETAC]. 2024. TRM Seed Zone Applications. Prineville, OR: U.S. Department of Agriculture, Forest Service, Western Wildland Environmental Threat Assessment Center. Available: https://www.fs.usda.gov/wwetac/threat-map/TRMSeedZoneMapper.php
USDA Natural Resources Conservation Service [USDA NRCS]. 2024. The PLANTS Database. Greensboro, NC: U.S. Department of Agriculture, Natural Resources Conservation Service, National Plant Data Team. https://plants.usda.gov/home
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2017. Seeds of Success collection data. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program.
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2023. Bureau of Land Management technical protocol for the collection, study, and conservation of seeds from native plant species for Seeds of Success. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program. 45 p.
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2024. Seeds of Success collection data. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program.
USDI Environmental Protection Agency [USDI EPA]. 2018. Ecoregions. Washington, DC: U.S. Environmental Protection Agency. https://www.epa.gov/eco-research/ecoregions
USDI Geological Survey [USDI USGS]. 2017. Biodiversity Information Serving Our Nation (BISON). U.S. Geological Survey. https://bison.usgs.gov/#home
Utah Crop Improvement Association [UCIA]. 2015. How to be a seed connoisseur. Logan, UT: UCIA, Utah Department of Agriculture and Food, Utah State University and Utah State Seed Laboratory. 16 p.
Welsh, S.L.; Atwood, N.D.; Goodrich, S.; Higgins, L.C., eds. 2015. A Utah Flora. Fifth Edition, revised. Provo, UT: Brigham Young University. 990 p.
Woolley, S.B., comp. 1936. Root systems of important range plants of the Boise River watershed: A catalogue of species excavated by Liter E. Spence [collaborator]. Unpublished paper on file at: U.S. Department of Agriculture, Forest Service, Intermountain Fire Sciences Lab, Missoula, MT. p. 59.
Wrobleski, D. 1999. Effects of prescribed fire on Wyoming big sagebrush communities: Implications for ecological restoration of sage grouse habitat. Corvallis, OR: Oregon State University. Thesis. 76 p.
Young, S.A.; Schrumpf, B.; Bouck, M.; Moore, M. 2020. How the Association of Official Seed Certifying Agencies (AOSCA) tracks wildland sourced seed and other plant propagating materials. Logan, UT: Utah State University, Utah Agricultural Experiment Station. 36 p.
How to Cite
Gucker, Corey L.; Shaw, Nancy L. 2024. Douglas’ dustymaiden (Chaenactis douglasii). In: Gucker, C.L.; Shaw, N.L., eds. Western forbs: Biology, ecology, and use in restoration. Reno, NV: Great Basin Fire Science Exchange. Online: https://westernforbs.org/